Abstract
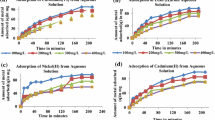
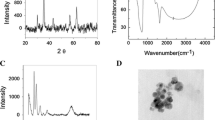
The isothermal absorption properties and kinetic model of Cr (VI) and Cr (III) onto ettringite were investigated using the batch adsorption method. IR analysis was used to study the difference and mechanism of the adsorption of chromium ions with different valence states. The results show that the adsorption of Cr(III) onto ettringite at 20 °C agrees with Langmuir’s isothermal model. The ion binding stability was significantly greater than that of Cr (VI). While the adsorption of Cr(VI) onto ettringite agrees with Freundlich’s isothermal model, the D-R model fits the adsorption isotherms of two types of valence Cr (R2> O.994). It can be concluded that the adsorption of Cr (III) onto ettringite is mainly by chemical adsorption and that the adsorption of Cr (VI) onto ettringite is mainly by physical adsorption. Dynamic model fitting and model parameter analyses show that the adsorption of Cr (III) onto ettringite agrees with the pseudo second order kinetics model given by Lagergren. The formation of chemical bonds is the main factor causing the fast adsorption. Cr (VI) adsorption is mainly dominated by liquid film diffusion, and the adsorption rate is much slower than that of Cr (III) adsorption.
Similar content being viewed by others
References
Shi Hui-sheng, Yuan Ling. Safety Assessment for Municipal Solid Wastes Incineration Fly Ashes-Cement Solidification Body[J]. Journal of Tongji, 2005, 33(3): 326–329
Wang Jiyuan. Research on Cement Solidification Experiment of Electroplation Heavy Metal Sludge[J]. Chemical Industry Times, 2006, 20(1): 44–47
Peng Chuan, Feng Qinge, Li Haoxuan. Leachablity and Speciation Analysis of Cr in Tannery Sludge after Cementation Treatment[J]. Bulletin of the Chinese Creamic Society, 2014, 33 (9): 2 205–2 211
Vempati RK, Mollah MYA, Chinthala AK, et al. Solidification/Stabilization of Toxic Metal Wastes Using Coke and Coal Combustion by-Products[J]. Waste Management, 1995, 15(5–6): 433–440
IkJunYeon, Soyoung, 이상우, 신택수, et al. The Solidification Characteristics of Recycled Aggregate Mixed with Incineration Ash and Waste Concrete[J]. Journal of the Korean Geoenvironmental Society, 2008, 9(5): 5–13
Qian GR, Hi J, Cao Y L, Xu YF, et al. Properties of MSW Fly Ash-calcium Sulfoaluminate Cement Matrix and Stabilization/Solidification on Heavy Metals[J]. Journal of Hazardous Materials, 2008, 152(1): 196–203
S Peysson, J Péra, M Chabannet. Immobilization of Heavy Metals by Calcium Sulfoaluminate Cement[J]. Cement and Concrete Research, 2005, 35 (12): 2 261–2 270
M L D Gougar, B E Scheetz, D M Roy. Ettringite and C-S—H Portland Cement Phases for Waste Iron Immobilization: A Review[J]. Waste Management, 1996, 16(4): 295–303
Bhishan Pandey, Stephen D, Kinrade, et al. Effects of Carbonation on the Leach Ability and Compressive Strength of Cement-Solidified and Geopolymer-Solidified Synthetic Metal Wastes[J]. Journal of Environmental Management, 2012, 101(30): 59–67
Klemm W A, Bhatty J I. Fixation of Heavy Metals as Oxyan- Ion-Substituted Ettringites[J]. Portland Cement Association, 2002: 9–10
Albino V, Cioffi R, Maroccoli M, et al. Potential Application of Ettringite Generating Systems for Hazardous Waste Stabilization[J]. Journal of Hazardous Material, 1996, 51(1–3): 241–252
Li XG, He C, Bai Y, et al. Stabilization/Solidification on Chromium (III) Wastes by C3A and C3A Hydrated Matrix[J]. Journal Of Hazardous Materials, 2014, 268: 61–67
Maria Chrysochoou, Dimitris Dermatas. Evaluation of Ettringite and Hydrocalumite Formation for Heavy Metal Immobilization: Literature Review and Experimental Study[J]. Journal of Hazardous Materials, 2006, 136(1): 20–33
Martin Vyšvařil, Patrik Bayer. Immobilization of Heavy Metals in Natural Zeolite-blended Cement Pastes[J]. Procedia Engineering, 2016, 151: 162–169
Zbigniew Giergiczny, Anna Król. Immobilization of Heavy Metals (Pb, Cu, Cr, Zn, Cd, Mn) in the Mineral Additions Containing Concrete Composites[J]. Hazardous Materials, 2008, 160(30): 247–255
R Berardi, R Cioffi, L Santoro. Matrix Stability and Leaching Behaviour in Ettringite-Based Stabilization Systems Doped with Heavy Metals[J]. Waste Management, 1998,17(8): 535–540
C A Luz, J Pera, M Cheriaf, et al. Behaviour of Calcium Sulfoaluminate Cement in Presence of High Concentrations of Chromium Salts[J]. Cement and Concrete Research, 2007, 37(4): 624–629
Nocuń-Wczelik Wiesława, Trybalska Barbara, Dziub Sylwia. The Properties of Cement Pastes and Mortars Processed with Some Heavy Metal Nitrates Containing Solutions[J]. Procedia Engineering, 2015, 108: 72–79
V Albino, R Cioffi, M Marroccoli, et al. Potential Application of Ettringite Generating Systems for Hazardous Waste Stabilization[J]. Journal of Hazardous Materials,1996, 51(1–3): 241–252
Jacobsen S D, Smyth JR, Swope R J. Thermal Expansion of Hydrated Six-Coordinate Silicon in the Umasite Ca3Si(OH)6(CO3) (SO4)•12H2O[J]. Phys. Chem. Minerals, 2003 (30): 321–329
Li Zongjin, Wei Xiaosheng, Li Wenlai. Preliminary Interpretat Ion of Hydration Process of Portland Cement Using Resistivity Measurement[J]. ACI Mater. J., 2003, 100(3): 253–257
Lan Junkang, Wang Yanxin. The Influence of SO4 2− on Compound Cement Solidified Deals With Cr (VI)[J]. Journal of the Chinese Ceramic Society, 2005, 33(10): 1 266–1 275
Maria Chrysochoou, Dimitris Dermatas. Evaluation of Ettringite and Hydrocalumite Formation for Heavy Metal Immobilization: Literature Review and Experimental Study[J]. Journal of Hazardous Materials, 2006, 136(1): 20–33
Srivastava P, Singh B, Angove M. Competitive Adsorption Behavior of Heavy Metals on Kaolinite[J]. Journal of Colloid and Interface Science, 2005, 290(1): 28–38
Behera S K, Kim J H, Guo X, et al. Adsorption Equilibrium and Kinetics of Polyvinyl Alcohol from Aqueous Solution on Powdered Activated Carbon[J]. Journal of Hazardous Materials, 2008, 153: 1 207–1 214
Rey F, Calle E., Casado J. Study of the Effects of Concentration and pH on the Dissociation Kinetics of Fe(II)-Fulvic Acid Complexes[J]. International Journal of Chemical Kinetics, 1998,30: 63–67
Namasivayam C, Jeyakumar R, Yamuna R T. Dye Removal from Waste-Water by Adsorption on Waste Fe(III)/Cr(III) Hydroxide[J]. Waste Management, 1994, 14(7): 643–648
Namasivayam C, Yamuna R T, Jayanthi J. Removal of Methylene Blue from Wastewater by Adsorption on Cellulosic Waste, Orange Peel[J]. Cellulose Chemistry and Technology, 2003, 37(7): 333–339
F A Banat, B Al-Bashir, S Al-Asheh, et al. Adsorption of Phenol by Bentonite[J]. Environmental Pollution, 2000, 107 (3): 391–398
Do D D. Adsorption Analysis: Equilibria and Kinetics[M]. London: Imperical College Press, 1998
Majumadar S S, Dad S K, Chakravaty, et al. Study on Lead Adsorption by Mucor Rouxii Biomass[J]. Desalination, 2010, 251: 96–102
Hameed B H, Salman J M, Ahamada A L. Adsorption Isotherm and Kinetic Modeling of 2, 4-D Pesticide on Activated Carbon Derived from Date Stones[J]. Journal of Hazardous Materials, 2009, 163: 121–126
Özcana A, Öncu E M, Özcan A S. Kinetics, Isotherm and the Dynamic Studies of Adsorption of Acid Blue 193 from Aqueous Solutions onto Natural Sepiolite[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2006, 277(1/2/3): 90–97
Gezginci M H, Timmermann B N. A Short Synthetic Route to Nordihydroguaiaretic Acid and Its Stereoisomer Using Ti-Induced Carbonyl-Coupling Reaction[J]. Tetrahedron Letters, 2001, 42(35): 6 083–6 085
Kandah M I. Zinc and Cadmium Adsorption on Low-Grade Phosphate[J]. Sep. Purif. Technol., 2004, 35(1): 61–70
F A Banat, B Al-Bashir, S Al-Asheh, et al. Adsorption of Phenol by Bentonite[J]. Environmental Pollution, 2000, 107(3): 391–398
Aharoni C D, Sparks L, Levinson S. Kinetics of Soil Chemical Reactions: Relationships Between Empirical Equations and Diffusion Models[J]. Soil Science Society of American Journal, 1991, 55: 1 307–1 310
Ho Y S. Citation Review of Lagergren Kinetic Rate Equationon Adsorption Reactions[J]. Scienctometrics, 2004, 9(1): 171–177
Gupta V K, Gupta M, Sharma S. Process Development for the Removal of Lead and Chromium from Aqueous Solutions Using Red-Mud-An Aluminium Industry Waste[J]. Water Research, 2001, 35(5): 1 125–1 134
Ho Y S, Mckay G. Pseudo-Second-Order Model for Sorption Process[J]. Process Biochemistry, 1999, 34(5): 451–465
Gupta V K, Rastogi A. Equilibrium and Kinetic Modeling of Cadmium(II) Biosorption by Nonliving Gal Biomass Oedo Gonium sp. From a Queous Phase[J]. Journal of Hazardous Materials, 2008, 153(1–2): 759–766
Sun Xiao-Li, Zeng Qing-xuan, Feng Chang-gen. Adsorption Kinetics of Chromium (VI) onto an Anion Exchange Fiber Containing Polyamine[J]. Acta Physico-Chimica Sinica, 2009, 25(10): 1 951–1 957
Ho Y S, McKay G. Pseudo-Second Order Model for Sorption Processes[J]. Process Biochem., 1999, 34(5): 451–465
A E Moor, H F W Taylor. Acta Crystallographica. Section B[M]. Malden: International Union of Crystallography, 1970
A M Cody, H Lee. The Effect of Environment on the Nucleation,Growth, and Stability of Ettringite {Ca2Al(OH)6]2·(SO4)3·26H2O[J]}. Cement and Concrete Research, 2004, 34: 869–881
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (No. 2010CB735803)
Rights and permissions
About this article
Cite this article
Wang, X., Cui, S., Yan, B. et al. Isothermal Adsorption Characteristics and Kinetics of Cr Ions onto Ettringite. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 34, 587–595 (2019). https://doi.org/10.1007/s11595-019-2092-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-019-2092-0