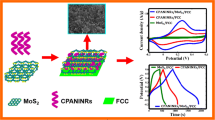
Abstract
In this work, polyaniline (PANI) nanorods were deposited on electro-etched carbon cloth (EC) by facile electrodeposition method with the existence of purified aniline and sulfuric acid. Various deposition potentials were applied to achieve a good electrochemical performance of EC-PANI electrode. Different applied potentials resulted in different morphologies of PANI deposits and studied by field emission scanning electron microscope (FESEM) and transmission electron microscope (TEM). X-ray diffraction (XRD) and Fourier transform infrared (FTIR) characterizations were used to confirm the deposition of PANI on the EC substrate. The optimized PANI nanorods electrode exhibited an excellent specific capacitance of 357.14 Fg−1 with an energy density of 40.18 Wh kg−1 and a power density of 1.28 Wkg−1 at a current density of 0.5 Ag−1 in 0.5 M H2SO4 electrolyte. A symmetrical cell of P1.4//PVA + 0.5 M H2SO4//P1.4 has recorded a good cycling stability with 95 and 88% capacitance retention at current densities of 200 and 300 mAg−1. EC-PANI electrode can be used as a scalable solution for high-performance energy storage devices.









Similar content being viewed by others
References
Cheng Q, Tang J, Ma J, Zhang H, Shinya N, Qin L-C (2011) Polyaniline-coated electro-etched carbon fiber cloth electrodes for supercapacitors. J Phys Chem C 115(47):23584–23590. https://doi.org/10.1021/jp203852p
Dirican M, Yanilmaz M, Zhang X (2014) Free-standing polyaniline-porous carbon nanofiber electrodes for symmetric and asymmetric supercapacitors. RSC Adv 4(103):59427–59435. https://doi.org/10.1039/C4RA09103E
Augustyn V, Simon P, Dunn B (2014) Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ Sci 7(5):1597–1614. https://doi.org/10.1039/C3EE44164D
Yan J, Wei T, Qiao W, Fan Z, Zhang L, Li T, Zhao Q (2010) A high-performance carbon derived from polyaniline for supercapacitors. Electrochem Commun 12(10):1279–1282. https://doi.org/10.1016/j.elecom.2010.06.037
Chen SM, Ramachandran R, Mani V, Saraswathi R (2014) Recent advancements in electrode materials for the high-performance electrochemical supercapacitors: a review. Int J Electrochem Sci 9(8):4072–4085
Xu M, Song Y, Ye Y, Gong C, Shen Y, Wang L, Wang L (2017) A novel flexible electrochemical glucose sensor based on gold nanoparticles/polyaniline arrays/carbon cloth electrode. Sensors Actuators B Chem 252:1187–1193. https://doi.org/10.1016/j.snb.2017.07.147
Peng H, Ma G, Sun K, Mu J, Zhou X, Lei Z (2015) A novel fabrication of nitrogen-containing carbon nanospheres with high rate capability as electrode materials for supercapacitors. RSC Adv 5(16):12034–12042. https://doi.org/10.1039/C4RA11889H
Jagadale AD, Dubal DP, Lokhande CD (2012) Electrochemical behavior of potentiodynamically deposited cobalt oxyhydroxide (CoOOH) thin films for supercapacitor application. Mater Res Bull 47(3):672–676. https://doi.org/10.1016/j.materresbull.2011.12.029
Chee WK, Lim HN, Zainal Z, Huang NM, Harrison I, Andou Y (2016) Flexible graphene-based supercapacitors: a review. J Phys Chem C 120(8):4153–4172. https://doi.org/10.1021/acs.jpcc.5b10187
Ramya R, Sivasubramanian R, Sangaranarayanan MV (2013) Conducting polymers-based electrochemical supercapacitors—progress and prospects. Electrochim Acta 101:109–129. https://doi.org/10.1016/j.electacta.2012.09.116
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sources 196(1):1–12. https://doi.org/10.1016/j.jpowsour.2010.06.084
Chen W, Rakhi RB, Alshareef HN (2013) Facile synthesis of polyaniline nanotubes using reactive oxide templates for high energy density pseudocapacitors. J Mater Chem A 1(10):3315–3324. https://doi.org/10.1039/C3TA00499F
Shown I, Ganguly A, Chen L-C, Chen K-H (2015) Conducting polymer-based flexible supercapacitor. Energy Sci Eng 3(1):2–26. https://doi.org/10.1002/ese3.50
Xinping H, Bo G, Guibao W, Jiatong W, Chun Z (2013) A new nanocomposite: carbon cloth based polyaniline for an electrochemical supercapacitor. Electrochim Acta 111:210–215. https://doi.org/10.1016/j.electacta.2013.07.226
Zhou G, Li F, Cheng H-M (2014) Progress in flexible lithium batteries and future prospects. Energy Environ Sci 7(4):1307–1338. https://doi.org/10.1039/C3EE43182G
Zhang L, Zhao G, Wang Y (2013) Polyaniline nanowire electrodes with high capacitance synthesized by a simple approach. Mater Sci Eng C 33(1):209–212. https://doi.org/10.1016/j.msec.2012.08.032
Zhao G-Y, Li H-L (2008) Preparation of polyaniline nanowire arrayed electrodes for electrochemical supercapacitors. Microporous Mesoporous Mater 110(2):590–594. https://doi.org/10.1016/j.micromeso.2007.06.023
Rusi, Majid SR (2013) Synthesis of MnO2 particles under slow cooling process and their capacitive performances. Mater Lett 108:69–71. https://doi.org/10.1016/j.matlet.2013.06.069
Zhou Z, Zhang X, Lu C, Lan L, Yuan G (2014) Polyaniline-decorated cellulose aerogel nanocomposite with strong interfacial adhesion and enhanced photocatalytic activity. RSC Adv 4(18):8966–8972. https://doi.org/10.1039/C3RA46441E
Guo F, Ye K, Huang X, Gao Y, Cheng K, Wang G, Cao D (2015) Palladium dispersed in three-dimensional polyaniline networks as the catalyst for hydrogen peroxide electro-reduction in an acidic medium. RSC Adv 5(114):94008–94015. https://doi.org/10.1039/C5RA19478D
Ding L, Li Q, Zhou D, Cui H, An H, Zhai J (2012) Modification of glassy carbon electrode with polyaniline/multi-walled carbon nanotubes composite: application to electro-reduction of bromate. J Electroanal Chem 668:44–50. https://doi.org/10.1016/j.jelechem.2011.12.018
Bláha M, Trchová M, Bober P, Morávková Z, Zujovic ZD, Filippov SK, Prokeš J, Pilař J, Stejskal J (2017) Structure and properties of polyaniline interacting with H-phosphonates. Synth Met 232:79–86. https://doi.org/10.1016/j.synthmet.2017.07.022
Tao S, Hong B, Kerong Z (2007) An infrared and Raman spectroscopic study of polyanilines co-doped with metal ions and H+. Spectrochim Acta A Mol Biomol Spectrosc 66(4):1364–1368. https://doi.org/10.1016/j.saa.2006.08.011
Trchová M, Šeděnková I, Konyushenko EN, Stejskal J, Holler P, Ćirić-Marjanović G (2006) Evolution of polyaniline nanotubes: the oxidation of aniline in water. J Phys Chem B 110(19):9461–9468. https://doi.org/10.1021/jp057528g
Venancio EC, Wang P-C, MacDiarmid AG (2006) The azanes: a class of material incorporating nano/micro self-assembled hollow spheres obtained by aqueous oxidative polymerization of aniline. Synth Met 156(5):357–369. https://doi.org/10.1016/j.synthmet.2005.08.035
Saranya K, Rameez M, Subramania A (2015) Developments in conducting polymer based counter electrodes for dye-sensitized solar cells – an overview. Eur Polym J 66:207–227. https://doi.org/10.1016/j.eurpolymj.2015.01.049
Patil DS, Pawar SA, Devan RS, Ron Ma Y, Ri Bae W, Hyeok Kim J, Patil PS (2014) Improved electrochemical performance of activated carbon/polyaniline composite electrode. Mater Lett 117:248–251. https://doi.org/10.1016/j.matlet.2013.11.129
Miao Y-E, Fan W, Chen D, Liu T (2013) High-performance supercapacitors based on hollow polyaniline nanofibers by electrospinning. ACS Appl Mater Interfaces 5(10):4423–4428. https://doi.org/10.1021/am4008352
Gómez H, Ram MK, Alvi F, Villalba P, Stefanakos E, Kumar A (2011) Graphene-conducting polymer nanocomposite as novel electrode for supercapacitors. J Power Sources 196(8):4102–4108. https://doi.org/10.1016/j.jpowsour.2010.11.002
Umeshbabu E, Rajeshkhanna G, Justin P, Rao GR (2015) Synthesis of mesoporous NiCo2O4–rGO by a solvothermal method for charge storage applications. RSC Adv 5(82):66657–66666. https://doi.org/10.1039/C5RA11239G
Adhikari H, Ghimire M, Ranaweera CK, Bhoyate S, Gupta RK, Alam J, Mishra SR (2017) Synthesis and electrochemical performance of hydrothermally synthesized Co3O4 nanostructured particles in presence of urea. J Alloys Compd 708:628–638. https://doi.org/10.1016/j.jallcom.2017.03.056
Randles JEB (1948) A cathode ray polarograph. Part II.-the current-voltage curves. Trans Faraday Soc 44(0):327–338. https://doi.org/10.1039/TF9484400327
Ma J, Tang S, Syed JA, Meng X (2016) Asymmetric hybrid capacitors based on novel bearded carbon fiber cloth-pinhole polyaniline electrodes with excellent energy density. RSC Adv 6(86):82995–83002. https://doi.org/10.1039/C6RA16291F
Wang L, Yang H, Pan G, Miao L, Chen S, Song Y (2017) Polyaniline-carbon nanotubes@ zeolite imidazolate framework67-carbon cloth hierarchical nanostructures for supercapacitor electrode. Electrochim Acta 240:16–23. https://doi.org/10.1016/j.electacta.2017.04.035
Zhang YX, Huang M, Li F, Wang XL, Wen ZQ (2014) One-pot synthesis of hierarchical MnO2-modified diatomites for electrochemical capacitor electrodes. J Power Sources 246:449–456. https://doi.org/10.1016/j.jpowsour.2013.07.115
Chen Y, Wang J-W, Shi X-C, Chen B-Z (2013) Pseudocapacitive characteristics of manganese oxide anodized from manganese coating electrodeposited from aqueous solution. Electrochim Acta 109:678–683. https://doi.org/10.1016/j.electacta.2013.07.174
Zhang H, Cao G, Wang W, Yuan K, Xu B, Zhang W, Cheng J, Yang Y (2009) Influence of microstructure on the capacitive performance of polyaniline/carbon nanotube array composite electrodes. Electrochim Acta 54(4):1153–1159. https://doi.org/10.1016/j.electacta.2008.09.004
Vello TP, de Oliveira RF, Silva GO, de Camargo DHS, Bufon CCB (2017) A simple capacitive method to evaluate ethanol fuel samples. Sci Rep 7:43432. https://doi.org/10.1038/srep43432 https://www.nature.com/articles/srep43432#supplementary-information
Zheng Y-Z, Ding H-Y, Zhang M-L (2009) Preparation and electrochemical properties of nickel oxide as a supercapacitor electrode material. Mater Res Bull 44(2):403–407. https://doi.org/10.1016/j.materresbull.2008.05.002
Funding
This project was funded by University Malaya Research Grant (FG034-AFR17).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Razali, S.A., Rusi & Majid, S.R. Fabrication of polyaniline nanorods on electro-etched carbon cloth and its electrochemical activities as electrode materials. Ionics 25, 2575–2584 (2019). https://doi.org/10.1007/s11581-018-2809-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2809-7