Abstract
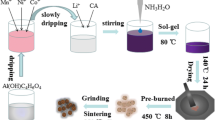
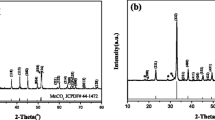
A series of layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode materials, of which manganese source was electrolytic manganese dioxide, with different contents of SO42− were successfully synthesized via ball-milling process and solid-state sintering method. The obtained materials were characterized by XRD, XPS, SEM-EDS, C-S, ICP, and HR-TEM. All the obtained materials presented well-ordered layered structure. When the content of SO42− was below 1.30 wt%, the electrochemical properties and structural stabilities at low rate for the layered materials with SO42− were not changed dramatically, while when the content of SO42− increased to 5.85 wt%, the initial discharge capacities decreased dramatically from 248.24 to 209.23 mAh g−1 at 10 mA g−1. And the pristine sample shows excellent cyclic property and rate capability. It delivered the discharge capacity of 175.25 mAh g−1 after 100 cycles with the highest capacity retention of 90.67% at 200 mA g−1. Particularly, the treated Li-rich Mn-based materials with the highest amount of SO42− exhibited the best cyclic stability and it delivers the highest capacity retention of 95.17% after 100 cycles at 200 mA g−1. However, its discharge capacities were much lower than the pristine material. As a result, the addition of SO42− could promote side reactions between electrode and electrolyte and deep-degree corrosion of electrode materials to affect the electrochemical properties and structural stabilities of the Li-rich Mn-based materials.









Similar content being viewed by others
Change history
23 July 2019
The authors regret to have uploaded the wrong Fig. 8 of this manuscript during the submission of the revised manuscript. The correct Fig. 8 is included here.
References
Li T, Li X, Wang Z, Guo H (2017) A short process for the efficient utilization of transition-metal chlorides in lithium-ion batteries: a case of Ni0.8Co0.1Mn0.1O1.1 and LiNi0.8Co0.1Mn0.1O2. J Power Sources 342:495–503
Wang J, Zhang G, Liu Z, Li H, Liu Y, Wang Z, Li X, Shih K, Mai L (2018) Li3V(MoO4)3 as a novel electrode material with good lithium storage properties and improved initial coulombic efficiency. Nano Energy 44:272–278
Li T, Li X, Wang Z, Guo H, Li Y, Wang J (2017) A new design concept for preparing nickel-foam-supported metal oxide microspheres with superior electrochemical properties. J Mater Chem A 5:13469–13474
Tan L, Li X, Wang Z, Guo H, Wang J (2018) Lightweight reduced graphene oxide@MoS2 interlayer as polysulfide barrier for high-performance lithium-sulfur batteries. ACS Appl Mater Interfaces 10:3707–3713
Zhou Y, Guo H, Yan G, Wang Z, Li X, Yang Z, Zheng A, Wang J (2018) Fluidized bed reaction towards crystalline embedded amorphous Si anode with much enhanced cycling stability. Chem Commun 54:3755–3758
Zhang Q, Chen H, Luo L, Zhao B, Luo H, Han X, Wang J, Wang C, Yang Y, Zhu T, Liu M (2018) Harnessing the concurrent reaction dynamics in active Si and Ge to achieve high performance lithium-ion batteries. Energy Environ Sci 11:669–681
Li L, Xu M, Yao Q, Chen Z, Song L, Zhang Z, Gao C, Wang P, Yu Z, Lai Y (2016) Alleviating surface degradation of nickel-rich layered oxide cathode material by encapsulating with nanoscale li-ions/electrons superionic conductors hybrid membrane for advanced li-ion batteries. ACS Appl Mater Interfaces 8(45):30879–30889
Zou Q, Liang Z, Du G-Y, Liu C-Y, Li EY, Lu Y-C (2018) Cation–directed selective polysulfide stabilization in alkali metal–sulfur batteries. J Am Chem Soc 140(34):10740–10748
Li L, Chen Z, Song L, Xu M, Zhu H, Gong L, Zhang K (2015) Characterization and electrochemical performance of lithium-active titanium dioxide inlaid LiNi0.5Co0.2Mn0.3O2 material prepared by lithium residue-assisted method. J Alloys Compd 638:77–82
Yan Z, Hu Q, Yan G, Li H, Shih K, Yang Z, Li X, Wang Z, Wang J (2017) Co3O4/Co nanoparticles enclosed graphitic carbon as anode material for high performance Li-ion batteries. Chem Eng J 321:495–501
Huang Z, Wang Z, Zheng X, Guo H, Li X, Jing Q, Yang Z (2015) Effect of Mg doping on the structural and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials. Electrochim Acta 182:795–802
Wu X, Li Y, Xiang Y, Liu Z, He Z, Wu X, Li Y, Xiong L, Li C, Chen J (2016) The electrochemical performance of aqueous rechargeable battery of Zn/Na0.44MnO2 based on hybrid electrolyte. J Power Sources 336:35–39
Arumugam D, Kalaignan GP, Vediappan K, Lee CW (2010) Synthesis and electrochemical characterizations of nano-scaled Zn doped LiMn2O4 cathode materials for rechargeable lithium batteries. Electrochim Acta 55:8439–8444
Zhao J, Wang Z, Guo H, Li X, He Z, Li T (2015) Synthesis and electrochemical characterization of Zn-doped Li-rich layered Li [Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material. Ceram Int 41:11396–11401
Chen H, Hu Q, Huang Z, He Z, Wang Z, Guo H, Li X (2016) Synthesis and electrochemical study of Zr-doped Li [Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode material for Li-ion battery. Ceram Int 42:263–269
Wang J, Liu Z, Yan G, Li H, Peng W, Li X, Song L, Shih K (2016) Improving the electrochemical performance of lithium vanadium fluorophosphate cathode material: focus on interfacial stability. J Power Sources 329:553–557
Wu L, Zhong S, Lv F, Liu J (2013) Improving the electrochemical performance of LiMnPO4/C by liquid nitrogen quenching. Mater Lett 110:38–41
Zhang Q, Liu Z, Zhao B, Cheng Y, Zhang L, Wu H-H, Wang M-S, Dai S, Zhang K, Ding D, Wu Y, Liu M (2018) Design and understanding of dendritic mixed-metal hydroxide nanosheets@N-doped carbon nanotube array electrode for high-performance asymmetric supercapacitors. Energy Storage Materials. https://doi.org/10.1016/j.ensm.2018.06.026
Zhang B, Ming L, Zheng J-c, Zhang J-f, Shen C, Han Y-d, Wang J-l, Qin S-e (2014) Synthesis and characterization of multi-layer core–shell structural LiFeBO3/C as a novel Li-battery cathode material. J Power Sources 261:249–254
Zhou L, Yin Z, Tian H, Ding Z, Li X, Wang Z, Guo H (2018) Spinel-embedded and Li3PO4 modified Li [Li0.2Mn0.54Ni0.13Co0.13]O2 cathode materials for high-performance Li-ion battries. Appl Surf Sci 456:763–770
Lee E-S, Manthiram A (2014) Smart design of lithium-rich layered oxide cathode compositions with suppressed voltage decay. J Mater Chem A 2:3932
Li H, Guo H, Wang Z, Wang J, Li X, Chen N, Gui W (2018) Improving rate capability and decelerating voltage decay of Li-rich layered oxide cathodes by chromium doping. Int J Hydrog Energy 43:11109–11119
Guo H, Li Q, He F, Li X, Wang Z, Peng W (2010) The role of sulfate ions coming from source materials on the properties of Li1.05Mn2O4 cathode for lithium ion batteries. Mater Chem Phys 124:922–926
Wu F, Li Q, Bao L, Zheng Y, Lu Y, Su Y, Wang J, Chen S, Chen R, Tian J (2018) Role of LaNiO3 in suppressing voltage decay of layered lithium-rich cathode materials. Electrochim Acta 260:986–993
Sathiya M, Abakumov AM, Foix D, Rousse G, Ramesha K, Saubanere M, Doublet ML, Vezin H, Laisa CP, Prakash AS, Gonbeau D, VanTendeloo G, Tarascon JM (2015) Origin of voltage decay in high-capacity layered oxide electrodes. Nat Mater 14:230–238
Li C, Zhong H, Wang S, Xue J (2014) Leaching behavior and risk assessment of heavy metals in a landfill of electrolytic manganese residue in Western Hunan, China. Hum Ecol Risk Assess Int J 20:1249–1263. https://doi.org/10.1080/10807039.2013.849482
Yu R, Lin Y, Huang Z (2015) Investigation on the enhanced electrochemical performances of Li1.2Ni0.13Co0.13Mn0.54O2 by surface modification with ZnO. Electrochim Acta 173:515–522
Zheng Z, Zao Y, Zhang Q, Cheng Y, Chen H, Zhang K, Wang M-S, Peng D-L (2018) Robust erythrocyte-like Fe2O3@carbon with yolk-shell structures as high-performance anode for lithium ion batteries. Chem Eng J 347:563–573
Liu Y, Wang Q, Wang X, Wang T, Gao Y, Su M, Dou A (2015) Improved electrochemical performance of Li1.2Ni0.2Mn0.6O2 cathode material with fast ionic conductor Li3VO4 coating. Ionics 21:2725–2733
Zheng Z, Guo X-D, Zhong Y-J, Hua W-B, Shen C-H, Chou S-L, Yang X-S (2016) Host structural stabilization of Li1.232Mn0.615Ni0.154O2 through K-doping attempt: toward superior electrochemical performances. Electrochim Acta 188:336–343
Li L, Xu M, Chen Z, Zhou X, Zhang Q, Zhu H, Wu C, Zhang K (2015) High-performance lithium-rich layered oxide materials: effects of chelating agents on microstructure and electrochemical properties. Electrochim Acta 174:446–455
Pan W, Peng W, Yan G, Guo H, Wang Z, Li X, Gui W, Wang J, Chen N (2018) Suppressing the voltage decay and enhancing the electrochemical performance of Li1.2Mn0.54Co0.13Ni0.13O2 by multifunctional Nb2O5 coating. Energ Technol. https://doi.org/10.1002/ente.201800253
Meng F, Guo H, Wang Z, Wang J, Li X, Li H, Wu X (2018) Magnesium-doped Li [Li0.2Mn0.54Ni0.13Co0.13]O2 cathode with high rate capability and improved cyclic stability. Ionics. https://doi.org/10.1007/s11581-018-2663-7
Liu Y, Zhang Z, Fu Y, Wang Q, Pan J, Su M, Battaglia VS (2016) Investigation the electrochemical performance of Li1.2Ni0.2Mn0.6O2 cathode material with ZnAl2O4 coating for lithium ion batteries. J Alloys Compd 685:523–532
Junkai Zhao, Zhixing Wang, Jiexi Wang, Huajun Guo, Xinhai Li, Weihua Gui, Ning Chen, Guochun Yan (2018) Anchoring K+ in Li+ sites of LiNi0.8Co0.15Al0.05O2 cathode material to suppress its structural degradation during high-voltage cycling. Energ Technol. https://doi.org/10.1002/ente.201800361
Mansoo Choi GH, Jin B-S (2014) Ultra-thin Al2O3 coating on the acid-treated 0.3Li2MnO3·0.7LiMn0.60Ni0.25Co0.15O2 electrode for Li-ion batteries. J Alloys Compd 608:110–117
Batini-Haberle I et al (2004) New class of potent catalysts of O2-dismutation. Mn (III) ortho-methoxyethylpyridyl- and di-ortho-methoxyethylimidazolylporphyrins. R Soc Chem 6,7(11):1696–1702. https://doi.org/10.1039/b400818a
Trimble RB, Ehrlich HL (1968) Bacteriology of manganese nodules III. Reduction of MnO2 by two strains of nodule bacteria. Appl Microbiol 16:695–702
Berlett BS, Chock PB, Yim MB, Stadtman ER (1989) Manganese (II) catalyzes the bicarbonate-dependent oxidation of amino acids by hydrogen peroxide and the amino acid-facilitated dismutation of hydrogen peroxide. PNAS 87(1):389–393
Zheng J, Wu X, Yang Y (2013) Improved electrochemical performance of Li [Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material by fluorine incorporation. Electrochim Acta 105:200–208
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 51574287, No. 51704332, No. 51674295 and No. 51674296), and the Program of Strategic Emerging Industries of Hunan Province, China (Grant No. 2017GK4019). This work received financial support from the collaborative Innovation center of Manganese-Zinc-Vanadium Industrial Technology (the 2011 plan of Hunan province). We also thank the Advanced Research Center of CSU for performing the HRTEM examination.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, F., Guo, H., Wang, Z. et al. The influences of SO42− from electrolytic manganese dioxide precursor on the electrochemical properties of Li-rich Mn-based material for Li-ion batteries. Ionics 25, 2585–2594 (2019). https://doi.org/10.1007/s11581-018-2796-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2796-8