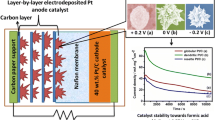
Abstract
The activity of bimetallic catalyst is predominantly determined by its composition and its shape. In this work, Pt–Pd bimetallic catalysts were codeposited using cyclic voltammetry on a carbon black-coated carbon paper at two different potential ranges (0 to 1.3 V and − 0.2 to 1.3 V vs. SHE) and with two different Pt precursors (H2PtCl6 and K2PtCl4). SEM analysis revealed that the deposit obtained from both K2PtCl4 and H2PtCl6 precursor resembled the shape of a flower-like dendrite when the deposition potential window was in the range of 0 to 1.3 V. However, shifting the lower potential limit from 0 to − 0.2 V resulted in a leaf-like dendritic structure, irrespective of the Pt precursor used. Leaf-like dendritic structures showed enhanced formic acid oxidation activity with high mass activity and superior stability compared to flower-like structures. The superior performance of the leaf-like structure was clearly evident from fuel cell polarization studies carried out at 70 °C, which showed a maximum power density of 49 mW cm−2, whereas flower-like structures showed a power density of 20 mW cm−2.











Similar content being viewed by others
References
Soloveichik GL (2014) Liquid fuel cells. Beilstein J Nanotechnol 5:1399–1418. https://doi.org/10.3762/bjnano.5.153
Rees NV, Compton RG (2011) Sustainable energy: a review of formic acid electrochemical fuel cells. J Solid State Electrochem 15:2095–2100. https://doi.org/10.1007/s10008-011-1398-4
Zhu Y, Ha SY, Masel RI (2004) High power density direct formic acid fuel cells. J Power Sources 130:8–14. https://doi.org/10.1016/j.jpowsour.2003.11.051
Rice C, Ha S, Masel RI, Waszczuk P, Wieckowski a, Barnard T (2002) Direct formic acid fuel cells. J Power Sources 111:83–89. https://doi.org/10.1016/S0378-7753(02)00271-9
Singh AK, Xu Q (2013) Synergistic catalysis over bimetallic alloy nanoparticles. ChemCatChem 5:652–676. https://doi.org/10.1002/cctc.201200591
Yu X, Pickup PG (2008) Recent advances in direct formic acid fuel cells (DFAFC). J Power Sources 182:124–132. https://doi.org/10.1016/j.jpowsour.2008.03.075
Kundu A, Jang JH, Gil JH, Jung CR, Lee HR, Kim S, Ku B, Oh YS (2007) Micro-fuel cells—current development and applications. J Power Sources 170:67–78. https://doi.org/10.1016/j.jpowsour.2007.03.066
Jiang K, Zhang H-X, Zou S, Cai W-B (2014) Electrocatalysis of formic acid on palladium and platinum surfaces: from fundamental mechanisms to fuel cell applications. Phys Chem Chem Phys 16:20360–20376. https://doi.org/10.1039/c4cp03151b
Ma C, Jin Y, Shi M, Chu Y, Xu Y, Jia W, Yuan Q, Chen J, Pan H, Dai Q (2014) Highly active Pd/WO3-CNTs catalysts for formic acid electrooxidation and study of the kinetics. Ionics 20:1419–1426. https://doi.org/10.1007/s11581-014-1100-9
Yang M, Zhu X, Tang Y, Wu P, Lu T (2015) Highly dispersed ultrafine palladium nanoparticles on three-dimensional mesoporous carbon for formic acid electro-oxidation. Ionics 21:2609–2614. https://doi.org/10.1007/s11581-015-1445-8
Ren M, Kang Y, He W, Zou Z, Xue X, Akins DL, Yang H, Feng S (2011) Origin of performance degradation of palladium-based direct formic acid fuel cells. Appl Catal B Environ 104:49–53. https://doi.org/10.1016/j.apcatb.2011.02.029
Łukaszewski M, Czerwin A (2006) Dissolution of noble metals and their alloys studied by electrochemical quartz crystal microbalance. J Electroanal Chem 589:38–45. https://doi.org/10.1016/j.jelechem.2006.01.007
Du C, Chen M, Wang W, Yin G (2011) Nanoporous PdNi alloy nanowires as highly active catalysts for the electro-oxidation of formic acid. ACS Appl Mater Interfaces 3:105–109. https://doi.org/10.1021/am100803d
Choi JH, Jeong KJ, Dong Y, Han J, Lim TH, Lee JS, Sung YE (2006) Electro-oxidation of methanol and formic acid on PtRu and PtAu for direct liquid fuel cells. J Power Sources 163:71–75. https://doi.org/10.1016/j.jpowsour.2006.02.072
Demirci UB (2007) Theoretical means for searching bimetallic alloys as anode electrocatalysts for direct liquid-feed fuel cells. J Power Sources 173:11–18. https://doi.org/10.1016/j.jpowsour.2007.04.069
Chen W, Kim J, Sun S, Chen S (2007) Composition effects of FePt alloy nanoparticles on the electro-oxidation of formic acid. Langmuir 23:11303–11310. https://doi.org/10.1021/la7016648
Kim Y, Kim HJ, Kim YS, Choi SM, Seo MH, Kim WB (2012) Shape- and composition-sensitive activity of Pt and PtAu catalysts for formic acid electrooxidation. J Phys Chem C 116:18093–18100. https://doi.org/10.1021/jp3054795
Wang R, Liao S, Ji S (2008) High performance Pd-based catalysts for oxidation of formic acid. J Power Sources 180:205–208. https://doi.org/10.1016/j.jpowsour.2008.02.027
Ghosh S, Raj CR (2015) Pt-Pd nanoelectrocatalyst of ultralow Pt content for the oxidation of formic acid: towards tuning the reaction pathway. J Chem Sci 127:949–957. https://doi.org/10.1007/s12039-015-0854-6
Obradović MD, Gojković SL (2014) Pd black decorated by Pt sub-monolayers as an electrocatalyst for the HCOOH oxidation. J Solid State Electrochem 18:2599–2607. https://doi.org/10.1007/s10008-014-2509-9
Zhang HX, Wang C, Wang JY, Zhai JJ, Bin CW (2010) Carbon-supported Pd-Pt nanoalloy with low Pt content and superior catalysis for formic acid electro-oxidation. J Phys Chem C 114:6446–6451. https://doi.org/10.1021/jp100835b
Porter NS, Wu H, Quan Z, Fang J (2013) Shape-control and electrocatalytic activity-enhancement of Pt-based bimetallic nanocrystals. Acc Chem Res 46:1867–1877
Nguyen VL, Ohtaki M, Matsubara T, Cao MT, Nogami M (2012) New experimental evidences of Pt-Pd bimetallic nanoparticles with core-shell configuration and highly fine-ordered nanostructures by high-resolution electron transmission microscopy. J Phys Chem C 116:12265–12274
Chu Y-Y, Wang Z-B, Jiang Z-Z, Gu D-M, Yin G-P (2012) Facile synthesis of hollow spherical sandwich PtPd/C catalyst by electrostatic self-assembly in polyol solution for methanol electrooxidation. J Power Sources 203:17–25. https://doi.org/10.1016/j.jpowsour.2011.11.025
Wang H, Xu C, Cheng F, Zhang M, Wang S, Jiang SP (2008) Pd/Pt core–shell nanowire arrays as highly effective electrocatalysts for methanol electrooxidation in direct methanol fuel cells. Electrochem Commun 10:1575–1578. https://doi.org/10.1016/j.elecom.2008.08.011
Yuan Q, Zhou Z, Zhuang J, Wang X (2010) Pd-Pt random alloy nanocubes with tunable compositions and their enhanced electrocatalytic activities. Chem Commun 46:1491–1493. https://doi.org/10.1039/b922792j
Zhang Z-C, Hui J-F, Guo Z-G, Yu Q-Y, Xu B, Zhang X, Liu Z-C, Xu C-M, Gao J-S, Wang X (2012) Solvothermal synthesis of Pt-Pd alloys with selective shapes and their enhanced electrocatalytic activities. Nano 4:2633–2639. https://doi.org/10.1039/c2nr12135b
Arjona N, Guerra-Balcazar M, Cuevas-Muniz FM, Alvarez-Contreras L, Ledesma-Garcia J, Arriaga LG (2013) Electrochemical synthesis of flower-like Pd nanoparticles with high tolerance toward formic acid electrooxidation. RSC Adv 3:15727–15733. https://doi.org/10.1039/C3RA41681J
Maniam KK, Chetty R (2015) Electrochemical synthesis of palladium dendrites on carbon support and their enhanced electrocatalytic activity towards formic acid oxidation. J Appl Electrochem 45:953–962. https://doi.org/10.1007/s10800-015-0860-x
Kim Y, Jung J, Kim S, Chae W (2013) Cyclic voltammetric and chronoamperometric deposition of CdS. Mater Trans 54:1467–1472
Cooper KR (2009) In situ PEM fuel cell electrochemical surface area and catalyst utilization measurement. Fuel Cell Mag 2:1–3. https://doi.org/10.1016/S1464-2859(00)80060-7
Barbir F (2013) Front Matter. In: PEM fuel cells theory and practice. Elsevier. https://doi.org/10.1016/B978-0-12-387710-9.01001-8
Muthukumar V, Chetty R (2017) Morphological transformation of electrodeposited Pt and its electrocatalytic activity towards direct formic acid fuel cells. J Appl Electrochem 47:735–745. https://doi.org/10.1007/s10800-017-1076-z
Ojani R, Hasheminejad E, Raoof JB (2014) Hydrogen evolution assisted electrodeposition of bimetallic 3D nano/micro-porous PtPd films and their electrocatalytic performance. Int J Hydrog Energy 39:8194–8203. https://doi.org/10.1016/j.ijhydene.2014.03.162
Plowman BJ, Jones LA, Bhargava SK (2015) Building with bubbles: the formation of high surface area honeycomb-like films via hydrogen bubble templated electrodeposition. Chem Commun 51:4331–4346. https://doi.org/10.1039/C4CC06638C
Yasin HM, Denuault G, Pletcher D (2009) Studies of the electrodeposition of platinum metal from a hexachloroplatinic acid bath. J Electroanal Chem 633:327–332. https://doi.org/10.1016/j.jelechem.2009.06.020
Peera SG, Sahu AK, Arunchander A, Nath K, Bhat SD (2015) Deoxyribonucleic acid directed metallization of platinum nanoparticles on graphite nano fibers as a durable oxygen reduction catalyst for polymer electrolyte fuel cells. J Power Sources 297:379–387. https://doi.org/10.1016/j.jpowsour.2015.08.009
Xu Y, Lin X (2007) Facile fabrication and electrocatalytic activity of Pt0.9Pd0.1 alloy film catalysts. J Power Sources 170:13–19. https://doi.org/10.1016/j.jpowsour.2007.03.064
Lee C-L, Chiou H-P (2012) Methanol-tolerant Pd nanocubes for catalyzing oxygen reduction reaction in H2SO4 electrolyte. Appl Catal B Environ 117–118:204–211. https://doi.org/10.1016/j.apcatb.2012.01.012
Meng H, Xie F, Chen J, Shen PK (2011) Electrodeposited palladium nanostructure as novel anode for direct formic acid fuel cell. J Mater Chem 21:11352–11358. https://doi.org/10.1039/c1jm10361j
Antolini E (2009) Palladium in fuel cell catalysis. Energy Environ Sci 2:915–931. https://doi.org/10.1039/b820837a
Taurino I, Sanzó G, Mazzei F, Favero G, De Micheli G, Carrara S (2015) Fast synthesis of platinum nanopetals and nanospheres for highly-sensitive non-enzymatic detection of glucose and selective sensing of ions. Sci Rep 5:15277–15287. https://doi.org/10.1038/srep15277
Xu W, Du D, Lan R, Humphreys J, Miller DN, Walker M, Wu Z, Irvine JTS, Tao S (2017) Electrodeposited NiCu bimetal on carbon paper as stable non-noble anode for efficient electrooxidation of ammonia. Appl Catal B Environ 3:1–9. https://doi.org/10.1016/j.apcatb.2016.11.003
Zhou Y, Neyerlin K, Olson TS, Pylypenko S, Bult J, Dinh HN, Gennett T, Shao Z, O’Hayre R (2010) Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports. Energy Environ Sci 3:1437–1446. https://doi.org/10.1039/c003710a
Watt-Smith MJ, Friedrich JM, Rigby SP, Ralph TR, Walsh FC (2008) Determination of the electrochemically active surface area of Pt/C PEM fuel cell electrodes using different adsorbates. J Phys D Appl Phys 41:174004–174011. https://doi.org/10.1088/0022-3727/41/17/174004
Zadick A, Dubau L, Demirci UB, Chatenet M (2016) Effects of Pd nanoparticle size and solution reducer strength on Pd/C electrocatalyst stability in alkaline electrolyte. J Electrochem Soc 163:F781–F787. https://doi.org/10.1149/2.0141608jes
Liu X-Y, Zhang Y, Gong M-X, Tang Y-W, Lu T-H, Chen Y, Lee J-M (2014) Facile synthesis of corallite-like Pt–Pd alloy nanostructures and their enhanced catalytic activity and stability for ethanol oxidation. J Mater Chem A 2:13840–13844. https://doi.org/10.1039/C4TA02522A
Lv J-J, Mei L-P, Weng X, Wang A-J, Chen L-L, Liu X-F, Feng J-J (2015) Facile synthesis of three-dimensional Pt–Pd alloyed multipods with enhanced electrocatalytic activity and stability for ethylene glycol oxidation. Nano 7:5699–5705. https://doi.org/10.1039/C5NR00174A
Jow J, Yang S, Chen H, Wu M, Ling T, Wei T (2009) Co-electrodeposition of Pt–Ru electrocatalysts in electrolytes with varying compositions by a double-potential pulse method for the oxidation of MeOH and CO. Int J Hydrog Energy 34:665–671. https://doi.org/10.1016/j.ijhydene.2008.11.032
El-Deab MS (2012) Platinum nanoparticles-manganese oxide nanorods as novel binary catalysts for formic acid oxidation. J Adv Res 3:65–71. https://doi.org/10.1016/j.jare.2011.04.002
Ji X, Lee KT, Holden R, Zhang L, Zhang J, Botton GA, Couillard M, Nazar LF (2010) Nanocrystalline intermetallics on mesoporous carbon for direct formic acid fuel cell anodes. Nat Chem 2:286–293. https://doi.org/10.1038/nchem.553
Maciá MD, Herrero E, Feliu JM (2003) Formic acid oxidation on Bi-Pt(111) electrode in perchloric acid media. A kinetic study. J Electroanal Chem 554–555:25–34. https://doi.org/10.1016/S0022-0728(03)00023-8
Maniam KK, Chetty R (2013) Electrodeposited palladium nanoflowers for electrocatalytic applications. Fuel Cells 13:1196–1204. https://doi.org/10.1002/fuce.201200162
Blair S, Lycke D, Coca I (2006) Palladium-platinum alloy anode catalysts for direct formic acid fuels. ECS Trans 3:1325–1332
Lee SJ, Mukerjee S, McBreen J, Rho YW, Kho YT, Lee TH (1998) Effects of Nafion impregnation on performances of PEMFC electrodes. Electrochim Acta 43:3693–3701. https://doi.org/10.1016/S0013-4686(98)00127-3
Myles TD, Kim S, Maric R, Mustain WE (2015) Application of a coated film catalyst layer model to a high temperature polymer electrolyte membrane fuel cell with low catalyst loading produced by reactive spray deposition technology. Catalysts 5:1673–1691. https://doi.org/10.3390/catal5041673
Fan X, Shi Y, Cui Y, Li D (2015) A facile electrochemical synthesis of three-dimensional porous Sn-Cu alloy/carbon nanotube nanocomposite as anode of high-power lithium-ion battery. Ionics 21:1909–1917. https://doi.org/10.1007/s11581-015-1372-8
Ren F, Wang H, Zhai C, Zhu M, Yue R, Du Y, Yang P, Xu J, Lu W (2014) Clean method for the synthesis of reduced graphene oxide-supported PtPd alloys with high electrocatalytic activity for ethanol oxidation in alkaline medium. ACS Appl Mater Interfaces 6:3607–3614. https://doi.org/10.1021/am405846h
Zhu C, Guo S, Dong S (2012) PdM (M = Pt, Au) bimetallic alloy nanowires with enhanced electrocatalytic activity for electro-oxidation of small molecules. Adv Mater 24:2326–2331. https://doi.org/10.1002/adma.201104951
Joo J, Uchida T, Cuesta A, Koper MTM, Osawa M (2014) The effect of pH on the electrocatalytic oxidation of formic acid/formate on platinum: a mechanistic study by surface-enhanced infrared spectroscopy coupled with cyclic voltammetry. Electrochim Acta 129:127–136. https://doi.org/10.1016/j.electacta.2014.02.04
Acknowledgements
The authors would like to thank Indian Institute of Technology (IIT) Madras for the financial support. We acknowledge the Department of Science and Technology, DST-FIST, for providing the instrumentation facility to the Department of Chemical Engineering, IIT Madras.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muthukumar, V., Chetty, R. Electrodeposited Pt–Pd dendrite on carbon support as anode for direct formic acid fuel cells. Ionics 24, 3937–3947 (2018). https://doi.org/10.1007/s11581-018-2526-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2526-2