Abstract
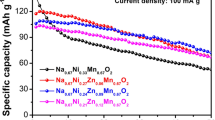
P2-Na0.67Ni0.33−x Cu x Mn0.67O2 (x = 0, 0.02, 0.04, 0.06, 0.08) cathode materials have been synthesized via acetate decomposition method. The elementary composition and crystal structure of the powders are studied in detail using inductively coupled plasma-atomic emission spectrometry (ICP-AES) and X-ray diffraction (XRD). XRD results demonstrate that Cu2+ ions have been incorporated into the crystal structure successfully and the P2-type structure remains unchanged after substitution. According to XPS data, Cu substitution does not change the valence states of Ni and Mn, whose predominant oxidation states in Na-Ni-Mn-O structure remains +2 and +4. The introduction of Cu2+ can effectively suppress P2-O2 phase transformation when charging to 4.5 V, and significantly improve rate performance and cyclic stability compared to the undoped material. The P2-Na0.67Ni0.27Cu0.06Mn0.67O2 sample can deliver an initial discharge capacity of 211.6 mAh g−1 at 10 mAh g−1 between 1.5 and 4.5 V, and a capacity retention of 93.9% after 10 cycles. Moreover, it can also deliver a discharge capacity of 115.2 mAh g−1 at 100 mAh g−1. In addition, electrochemical impedance spectroscopy (EIS) reveals that P2-Na0.67Ni0.27Cu0.06Mn0.67O2 cathode exhibits a higher electronic conductivity and faster sodium ion diffusion velocity than that of undoped sample. These results show that P2-Na0.67Ni0.27Cu0.06Mn0.67O2 is a promising high-voltage cathode material for sodium-ion batteries.







Similar content being viewed by others
References
Yuan X, Xu QJ, Wang C, Liu X, Liu H, Xia Y (2015) A facile and novel organic coprecipitation strategy to prepare layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 with high capacity and excellent cycling stability. J Power Sources 279:157–164
Xu H, Zong J, Chen S, Ding F, Lu ZW, Liu XJ (2016) Synthesis and evaluation of Na[Ni0.5Co0.2Mn0.3]O2 as a cathode material for Na-ion battery. Ceram Int 42:12521–12524
Guo H, Wang Y, Han W, Yu Z, Qi X, Sun K, Hu YS, Liu Y, Chen D, Chen L (2015) Na-deficient O3-type cathode material Na0.8[Ni0.3Co0.2Ti0.5]O2 for room-temperature sodium-ion batteries. Electrochim Acta 158:258–263
Wen Y, Wang B, Zeng G, Nogita K, Ye D, Wang L (2015) Electrochemical and structural study of layered P2-type Na2/3[Ni1/3Mn2/3]O2 as cathode material for sodium-ion battery. Chem Asian J 10:661–666
Lu Z, Dahn JR (2001) In situ X-ray diffraction study of P2-Na2/3[Ni1/3Mn2/3]O2. J Electrochem Soc 148:A1225
Wang H, Yang B, Liao XZ, Xu J, Yang D, He YS, Ma ZF (2013) Electrochemical properties of P2-Na2/3[Ni1/3Mn2/3]O2 cathode material for sodium ion batteries when cycled in different voltage ranges. Electrochim Acta 113:200–204
Wu X, Guo J, Wang D, Zhong G, McDonald MJ, Yang Y (2015) P2-type Na0.66[Ni0.33–xZnxMn0.67]O2 as new high-voltage cathode materials for sodium-ion batteries. J Power Sources 281:18–26
Billaud J, Singh G, Armstrong AR, Gonzalo E, Roddatis V, Armand M, Rojo T, Bruce PG (2014) Na0.67Mn1-xMgxO2 (0≦x≦0.2): a high capacity cathode for sodium-ion batteries. Energy Environ Sci 7:1387–1391
Sharma N, Tapiaruiz N, Singh G, Armstrong AR, Pramudita JC, Brand HEA, Billaud J, Bruce PG, Rojo T (2015) Rate dependent performance related to crystal structure evolution of Na0.67Mn0.8Mg0.2O2 in a sodium-ion battery. Chem Mater 27:6976–6986
Li ZY, Gao R, Zhang J, Zhang X, Hu Z, Liu X (2016) New insights into designing high-rate performance cathode materials for sodium ion batteries by enlarging the slab-spacing of the Na-ion diffusion layer. J Mater Chem A 4:3453–3461
Zhang XH, Pang WL, Wan F, Guo JZ, Lü HY, Li JY, Wu XL (2016) P2-Na2/3Ni1/3Mn5/9Al1/9O2 microparticles as superior cathode material for sodium-ion batteries: enhanced properties and mechanism via graphene connection. ACS Appl Mater Interfaces 8:20650–20659
Hasa I, Buchholz D, Passerini S, Scrosati B, Hassoun J (2014) High performance Na0.5[Ni0. 23Fe0.13Mn0.63] O2 cathode for sodium-ion batteries. Adv Energy Mater 4:140083
Zhao W, Kirie H, Tanaka A, Unno M, Yamamoto S, Noguchi H (2014) Synthesis of metal ion substituted P2-Na2/3[Ni1/3Mn2/3]O2 cathode material with enhanced performance for Na ion batteries. Mater Lett 135:131–134
Kubota K, Yoshida H, Yabuuchi N (2014) P2-type Na2/3[Ni1/3Mn2/3-xTix]O2 as a 3.7 V class positive electrode for Na-ion batteries
Li Y, Yang Z, Xu S, Mu L, Gu L, Hu YS, Chen L (2015) Air-stable copper-based P2-Na7/9Cu2/9Fe1/9Mn2/3O2 as a new positive electrode material for sodium-ion batteries. Adv Sci 2:150031
Kang W, Zhang Z, Lee PK, Ng TW, Li W, Tang Y, Yu DYW (2015) Copper substituted P2-type Na0.67CuxMn1-xO2: a stable high-power sodium-ion battery cathode. J Mater Chem A 3:22846–22852
Komaba S, Yoshii K, Ogata A, Nakai I (2009) Structural and electrochemical behaviors of metastable Li2/3[Ni1/3Mn2/3]O2 modified by metal element substitution. Electrochim Acta 54:2353–2359
Lee DH, Xu J, Meng YS (2013) An advanced cathode for Na-ion batteries with high rate and excellent structural stability. Phys Chem Chem Phys 15:3304–3312
Pan L, Xia Y, Qiu B, Zhao H, Guo H, Jia K, Gu Q, Liu Z (2016) Structure and electrochemistry of B doped Li[Li0.2Ni0.13Co0.13Mn0.54]1-xBxO2 as cathode materials for lithium-ion batteries. J Power Sources 327:273–280
Hu G, Zhang M, Liang L, Peng Z, Du K, Cao Y (2016) Mg–Al–B co-substitution Li[Ni0.5Co0.2Mn0.3]O2 cathode materials with improved cycling performance for lithium-ion battery under high cutoff voltage. Electrochim Acta 190:264–275
Ruan Z, Zhu Y, Teng X (2015) Effect of pre-thermal treatment on the lithium storage performance of Li[Ni0.8Co0.15Al0.05]O2. J Mater Sci 51:1400–1408
Zhang Y, Ye K, Cheng K, Wang G, Cao D (2014) Three-dimensional lamination-like P2-Na2/3[Ni1/3Mn2/3]O2 assembled with two-dimensional ultrathin nanosheets as the cathode material of an aqueous capacitor battery. Electrochim Acta 148:195–202
Lee JYK, Jahng JW (2014) Highly palatable food during adolescence improves anxiety-like behaviors and hypothalamic-pituitary-adrenal axis dysfunction in rats that experienced neonatal maternal separation. Endocrinol Metab 29:169
Liu Z, Zhou H, Ang SS, Zhang JJ (2016) Evaluation of low-cost natrochalcite Na[Cu2(OH)(H2O)(SO4)2] as an anode material for Li- and Na-ion batteries. Electrochim Acta 211:619–626
Gopukumar S, Chung KY, Kim KB (2004) Novel synthesis of layered Li[Ni1/2Mn1/2]O2 as cathode material for lithium rechargeable cells. Electrochim Acta 49:803–810
Jian Z, Yu H, Zhou H (2013) Designing high-capacity cathode materials for sodium-ion batteries. Electrochem Commun 34:215–218
Han E, Jing Q, Zhu L, Zhang G, Ma S (2015) The effects of sodium additive on Li1.17[Ni0.10Co0.10Mn0.63]O2 for lithium ion batteries. J Alloys Compd 618:629–634
Wang Y, Xiao R, Hu YS, Avdeev M, Chen L (2015) P2-Na0.6[Cr0.6Ti0.4]O2 cation-disordered electrode for high-rate symmetric rechargeable sodium-ion batteries. Nat Commun 6:6954
Buchholz D, Chagas LG, Winter M, Passerini S (2013) P2-type layered Na0.45[Ni0.22Co0.11Mn0.66]O2 as intercalation host material for lithium and sodium batteries. Electrochim Acta 110:208–213
Komaba S, Yabuuchi NNT, Ogata A, Ishikawa T, Nakai I (2012) Study on the reversible electrode reaction of Na1-x[Ni0.5Mn0.5]O2 for a rechargeable sodium-ion battery. Inorg Chem 51:6211–6220
Clément RJ, Bruce PG, Grey CP (2015) Review—manganese-based P2-type transition metal oxides as sodium-ion battery cathode materials. J Electrochem Soc 162:A2589–A2604
Shi SJ, Tu JP, Tang YY, Yu YX, Zhang YQ, Wang XL, Gu CD (2013) Combustion synthesis and electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 with improved rate capability. J Power Sources 228:14–23
Shanmugam R, Lai W (2014) Study of transport properties and interfacial kinetics of Na2/3[Ni1/3MnxTi2/3-x]O2 (x=0,1/3) as electrodes for Na-ion batteries. J Electrochem Soc 162:A8–A14
Molenda J, Ojczyk W, Marzec J (2007) Electrical conductivity and reaction with lithium of LiFe 1−y MnyPO4 olivine-type cathode materials. J Power Sources 174:689–694
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, S., Han, E., Xu, H. et al. P2-type Na0.67Ni0.33−x Cu x Mn0.67O2 as new high-voltage cathode materials for sodium-ion batteries. Ionics 23, 3057–3066 (2017). https://doi.org/10.1007/s11581-017-2122-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2122-x