Abstract
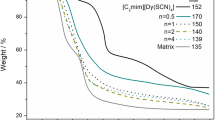
Hexanoyl chitosan and lauroyl chitosan were prepared by acyl modification of chitosan. Films of hexanoyl chitosan- and lauroyl chitosan-based polymer electrolytes incorporated with different weight concentrations of sodium iodide (NaI) were prepared using the solution casting technique. FTIR and differential scanning calorimetry (DSC) results suggested that NaI interacted with both hexanoyl chitosan and lauroyl chitosan. Maximum conductivities of 1.3 × 10−6 and 1.1 × 10−8 S cm−1 are achieved for hexanoyl chitosan and lauroyl chitosan, respectively. Higher conductivity in hexanoyl chitosan is attributed to higher ion mobility as supported by DSC results. The dielectric constants of neat hexanoyl chitosan and lauroyl chitosan are 2.7 and 1.9, respectively, estimated from impedance spectroscopy. Higher dielectric constant of hexanoyl chitosan resulted in greater NaI dissociation and hence higher conductivity. Deconvolution of O═C-NHR and OCOR bands of polymer has been carried out to estimate the amount of dissociated Na+ ions from NaI. The findings were in good agreement with conductivity results. In order to assess quantitatively, the conductivity, parameter number, n, and mobility, μ, of ions were calculated using impedance spectroscopy. XRD results showed the influence of NaI on the crystalline content of the electrolyte system. Sample with lower crystalline content exhibited higher conductivity.















Similar content being viewed by others
References
Alves R, Donoso JP, Magon CJ, Silva IDA, Pawlicka A, Silva MM (2015) Solid polymer electrolytes based on chitosan and europium triflate. J Non-Cryst Solids 432 part B:307–312
Rudhziah S, Ahmad A, Ahmad I, Mohamed NS (2015) Biopolymer electrolytes based on blend of kappa-carrageenan and cellulose derivatives for potential application in dye sensitized solar cell. Electrochim Acta 175:162–168
Majid SR, Sabadini RC, Kanicki J, Pawlicka A (2014) Impedance analysis of gellan gum-poly(vinyl pyrrolidone) membranes. Mol Cryst Liq Cryst 604:84–95
Woo HJ, Majid SR, Arof AK (2012) Dielectric properties and morphology of polymer electrolyte based on poly(ɛ-caprolactone) and ammonium thiocyanate. Mater Chem Phys 134:755–761
Aziz SB, Abidin ZHZ (2014) Electrical and morphological analysis of chitosan:AgTf solid electrolyte. Mater Chem Phys 144:280–286
Winie T, Majid SR, Khiar ASA, Arof AK (2006) Ionic conductivity of chitosan membrane and application for electrochemical devices. Polym Adv Technol 17:523–527
Moore G, Goerge A (1981) Reactions of chitosan: 2. Preparation and reactivity of N-acyl derivatives of chitosan. Int J Biol Micromol 3:292–296
Zong Z, Kimura Y, Takahashi M, Yamane H (2000) Characterization of chemical and solid state structures of acylated chitosans. Polymer 41:899–906
Nishimura S, Kohgo O, Kurita K, Kuzuhara H (1991) Chemospecific manipulations of a rigid polysaccharide: syntheses of novel chitosan derivatives with excellent solubility in common organic solvents by regioselective chemical modifications. Macromolecules 24:4745–4748
Yalpani M, Hall LD (1984) Some chemical and analytical aspects of polysaccharide modifications. III. Formation of branched-chain, soluble chitosan derivatives. Macromolecules 17:272–281
Winie T, Arof AK (2006) Hexanoyl chitosan-based gel electrolyte for use in lithium-ion cell. Polym Adv Technol 17:552–555
Rosli NHA, Chan CH, Subban RHY, Winie T (2012) Studies on the structural and electrical properties of hexanoyl chitosan/polystyrene-based polymer electrolytes. Phys Procedia 25:215–220
Winie T, Shahril NSM, Chan CH, Arof AK (2014) Selective localization of lithium trifluoromethanesulfonate in the blend of hexanoyl chitosan and polystyrene. High Perform Polym 26:666–671
Winie T, Shahril NSM (2015) Conductivity enhancement by controlled percolation of inorganic salt in multiphase hexanoyl chitosan/polystyrene polymer blends. Front Mater Sci 9:132–140
Winie T, Rosli NHA, Ahmad MR, Subban RHY, Chan CH (2012) TiO2 dispersed hexanoyl chitosan-polystyrene- LiCF3SO3 composite electrolyte characterized for electrical and tensile properties. Polym Res J 7:171–181
Winie T, Arof AK (2014) Impedance spectroscopy: basic concepts and application for electrical evaluation of polymer electrolytes. In: Impedance Spectroscopy, 1st edn. Apple Academic Press, USA, pp 335–363
Linford RG (1988) In: Chowdari BVR, Radhakrishna S (eds) Solid state Ionics devices. World Scientific, Singapore, pp 551–571
Arof AK, Amirudin S, Yusof SZ, Noor IM (2014) A method based on impedance spectroscopy to determine transport properties of polymer electrolytes. R Soc Chem 16:1856–1867
Sim LN, Majid SR, Arof AK (2012) FTIR studies of PEMA/PVdF-HFP blend polymer electrolyte system incorporated with LiCF3SO3 salt. Vib Spectrosc 58:57–66
Sharma JP, Sekhon SS (2006) PMMA-based polymer gel electrolytes containing NH4PF6: role of molecular weight of polymer. Mater Sci Eng B 129:104–108
Bohnke O, Frand G, Resrazi M, Rousseelet C, Truche C (1993) Fast ion transport in new lithium electrolytes gel with PMMA. Influence of polymer concentration. Solid State Ionics 66:97
Kumar R, Sekhon SS (2008) Effect of molecular weight of PMMA on the conductivity and viscosity behavior of polymer gel electrolytes containing. Ionics 14:509–514
Chandra S, Tolpadi SK, Hashmi SA (1988) Transient ionic current measurement of ionic mobilities in a few proton conductors. Solid State Ionics 28–30:651–655
Williamson MJ, Southhall JP, Hubbard VSA (1998) NMR measurements of ionic mobility in model polymer electrolyte solutions. Electrochim Acta 43:1415–1420
Winie T, Arof AK (2004) Dielectric behaviour and AC conductivity of LiCF3SO3 doped H-chitosan polymer films. Ionics 10:193–199
Acknowledgements
The authors wish to thank Universiti Teknologi MARA and Ministry of Higher Education Malaysia for supporting this work through grant FRGS/1/2014/SG06/UiTM/02/1. F.H. Muhammad thanks the University for the Scholarship Award and Assoc. Prof. Dr. Halimah Mohamed Kamari from Universiti Putra Malaysia for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muhammad, F., Jamal, A. & Winie, T. Study on factors governing the conductivity performance of acylated chitosan-NaI electrolyte system. Ionics 23, 3045–3056 (2017). https://doi.org/10.1007/s11581-017-2118-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2118-6