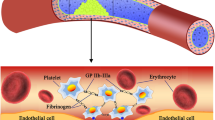
Abstract
The regions with high non-physiological shear stresses (NPSS) are inevitable in blood-contacting medical devices (BCMDs) used for mechanically assisted circulatory support. NPSS can cause platelet activation and receptor shedding potentially resulting in the alteration of hemostatic function. In this study, we developed a dissipative particle dynamics model to characterize clot formation (platelet–collagen and inter-platelet adhesion) of NPSS-traumatized blood at a vascular injury site. A rectangular tube of 50 × 50 × 200 µm with an 8 × 8 µm collagen-coated area was modeled as a small blood vessel and perfusion with blood. Clot formation dynamics during perfusion was simulated. NPSS-traumatized blood was modeled to have more activated platelet and fewer adhesion receptors with weakened inter-platelet binding. Computational results showed that clots grew at a faster rate while the structure of the clots was less stable and collapsed more frequently for NPSS-traumatized blood compared with normal blood. The finding that NPSS-traumatized platelets could result in quicker but more easily breakable blood clots at injury sites may explain why increased risks of thrombotic and bleeding complications occurred concurrently in patients implanted with BCMDs.








Similar content being viewed by others
References
Affeld K, Goubergrits L, Kertzscher U, Gadischke J, Reininger A (2004) Mathematical model of platelet deposition under flow conditions. Int J Artif Organs 27:699–708
Arthur J, Dunkley S, Andrews R (2007) Platelet glycoprotein VI-related clinical defects. Br J Haematol 139:363–372
Balcioglu O, Engin C, Yagdi T, Nalbantgil S, Baysal B, Erkul S, Engin Y, Kutlayey I, Ozbaran M (2013) Effect of aortic valve movements on gastrointestinal bleeding that occurred in continuous flow left ventricular assist device patients. Transpl Proc 45:1020–1021. https://doi.org/10.1016/j.transproceed.2013.02.072
Britton S, Kim O, Pancaldi F, Xu Z, Litvinov RI, Weisel JW, Alber M (2019) Contribution of nascent cohesive fiber–fiber interactions to the non-linear elasticity of fibrin networks under tensile load. Acta Biomater 94:514–523. https://doi.org/10.1016/j.actbio.2019.05.068
Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H (2011) Platelets at work in primary hemostasis. Blood Rev 25:155–167. https://doi.org/10.1016/j.blre.2011.03.002
Casa LDC, Ku DN (2017) Thrombus formation at high shear rates. Annu Rev Biomed Eng 19:415–433. https://doi.org/10.1146/annurev-bioeng-071516-044539
Chen Z, Mondal NK, Ding J, Gao J, Griffith BP, Wu ZJ (2015a) Shear-induced platelet receptor shedding by non-physiological high shear stress with short exposure time: glycoprotein Ibalpha and glycoprotein VI. Thromb Res 135:692–698. https://doi.org/10.1016/j.thromres.2015.01.030
Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Griffith BP, Wu ZJ (2015b) Activation and shedding of platelet glycoprotein IIb/IIIa under non-physiological shear stress. Mol Cell Biochem 409:93–101. https://doi.org/10.1007/s11010-015-2515-y
Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Wu ZJ (2016) Paradoxical effect of nonphysiological shear stress on platelets and von Willebrand Factor. Artif Organs 40:659–668. https://doi.org/10.1111/aor.12606
Chen Z, Mondal NK, Zheng S, Koenig SC, Slaughter MS, Griffith BP, Wu ZJ (2017) High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets. https://doi.org/10.1080/09537104.2017.1384542
Chen Z, Zhang J, Kareem K, Tran D, Conway RG, Arias K, Griffith BP, Wu ZJ (2019) Device-induced platelet dysfunction in mechanically assisted circulation increases the risks of thrombosis and bleeding. Artif Organs. https://doi.org/10.1111/aor.13445
Coffman JD, Lempert JA (1975) Venous flow velocity, venous volume and arterial blood flow. Circulation 52:141–145. https://doi.org/10.1161/01.cir.52.1.141
Cranmer SL, Ashworth KJ, Yao Y, Berndt MC, Ruggeri ZM, Andrews RK, Jackson SP (2011) High shear-dependent loss of membrane integrity and defective platelet adhesion following disruption of the GPIbalpha–filamin interaction. Blood 117:2718–2727. https://doi.org/10.1182/blood-2010-07-296194
Crow S, Chen D, Milano C, Thomas W, Joyce L, Piacentino V III, Sharma R, Wu J, Arepally G, Bowles D, Rogers J, Villamizar-Ortiz N (2010a) Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thor Surg 90:1263–1269. https://doi.org/10.1016/j.athoracsur.2010.04.099(Discussion 1269)
Crow S, Milano C, Joyce L, Chen D, Arepally G, Bowles D, Thomas W, Ortiz NV (2010b) Comparative analysis of von Willebrand factor profiles in pulsatile and continuous left ventricular assist device recipients. ASAIO J 56:441–445. https://doi.org/10.1097/MAT.0b013e3181e5de0a
Eckstein EC, Belgacem F (1991) Model of platelet transport in flowing blood with drift and diffusion terms. Biophys J 60:53–69
Español P, Warren P (1995) Statistical mechanics of dissipative particle dynamics. Europhys Lett 30:191–196. https://doi.org/10.1209/0295-5075/30/4/001
Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B (2002) Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med 8:1175–1181. https://doi.org/10.1038/nm782
Goda M, Jacobs S, Rega F, Peerlinck K, Jacquemin M, Droogne W, Vanhaecke J, Van Cleemput J, Van den Bossche K, Meyns B (2013) Time course of acquired von Willebrand disease associated with two types of continuous-flow left ventricular assist devices: HeartMate II and CircuLite Synergy Pocket Micro-pump. J Heart Lung Transpl 32:539–545. https://doi.org/10.1016/j.healun.2013.02.006
Goodman PD, Barlow ET, Crapo PM, Mohammad SF, Solen KA (2005) Computational model of device-induced thrombosis and thromboembolism. Ann Biomed Eng 33:780–797
Groot RD, Warren PB (1997) Dissipative particle dynamics: bridging the gap between atomistic and mesoscopic simulation. J Chem Phys 107:4423–4435. https://doi.org/10.1063/1.474784
Gupta P, Zhang P, Sheriff J, Bluestein D, Deng Y (2019) A multiscale model for recruitment aggregation of platelets by correlating with in vitro results. Cell Mol Bioeng 12:327–343. https://doi.org/10.1007/s12195-019-00583-2
Heilmann C, Geisen U, Beyersdorf F, Nakamura L, Trummer G, Berchtold-Herz M, Schlensak C, Zieger B (2011) Acquired von Willebrand syndrome is an early-onset problem in ventricular assist device patients. Eur J Cardio Thor Surg 40:1328–1333. https://doi.org/10.1016/j.ejcts.2011.03.021(Discussion 1233)
Jackson SP (2007) The growing complexity of platelet aggregation. Blood 109:5087–5095. https://doi.org/10.1182/blood-2006-12-027698
Kamada H, Tsubota K, Nakamura M, Wada S, Ishikawa T, Yamaguchi T (2010) A three-dimensional particle simulation of the formation and collapse of a primary thrombus. Int J Numer Methods Biomed Eng 26:488–500. https://doi.org/10.1002/cnm.1367
Kirklin JK, Naftel DC, Kormos RL et al (2013) Fifth INTERMACS annual report: risk factor analysis from more than 6000 mechanical circulatory support patients. J Heart Lung Transpl 32:141–156
Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL (1996) Platelets and shear stress. Blood 88:1525–1541
Leytin V, Allen DJ, Mykhaylov S, Mis L, Lyubimov EV, Garvey B, Freedman J (2004) Pathologic high shear stress induces apoptosis events in human platelets. Biochem Biophys Res Commun 320:303–310. https://doi.org/10.1016/j.bbrc.2004.05.166
Lippok S, Radtke M, Obser T, Kleemeier L, Schneppenheim R, Budde U, Netz RR, Radler JO (2016) Shear-induced unfolding and enzymatic cleavage of full-length VWF multimers. Biophys J 110:545–554. https://doi.org/10.1016/j.bpj.2015.12.023
Liu MB, Liu GR, Zhou LW, Chang JZ (2015) Dissipative particle dynamics (DPD): an overview and recent developments. Arch Comput Methods Eng 22:529–556. https://doi.org/10.1007/s11831-014-9124-x
López J, Andrews R, Afshar-Kharghan V, Berndt M (1998) Bernard-Soulier syndrome. Blood 91:4397–4418
Makdisi G, Wang IW (2015) Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thor Dis 7:E166–E176. https://doi.org/10.3978/j.issn.2072-1439.2015.07.17
Martys NS, Mountain RD (1999) Velocity Verlet algorithm for dissipative-particle-dynamics-based models of suspensions. Phys Rev E 59:3733–3736. https://doi.org/10.1103/PhysRevE.59.3733
Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, Tatooles AJ, Moazami N, Kormos RL, Hathaway DR, Najarian KB, Bhat G, Aaronson KD, Boyce SW, Investigators H.B.T.T.A.T. (2014) An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 33:23–34. https://doi.org/10.1016/j.healun.2013.12.001
Plimpton S, Crozier P, Thompson A (2008) LAMMPS-large-scale atomic/molecular massively parallel simulator. IEEE, Seattle
Sorensen E, Burgreen GW, Wagner WR, Antaki JF (1999a) Computational simulation of platelet deposition and activation: I. Model development and properties. Ann Biomed Eng 27:436–448
Sorensen EN, Burgreen GW, Wagner WR, Antaki JF (1999b) Computational simulation of platelet deposition and activation: II. Results for Poiseuille flow over collagen. Ann Biomed Eng 27:449–458
Starling RC, Blackstone EH, Smedira NG (2014) Increase in left ventricular assist device thrombosis. N Engl J Med 370:1465–1466. https://doi.org/10.1056/NEJMc1401768
Tomita A, Tamura N, Nanazawa Y, Shiozaki S, Goto S (2015) Development of virtual platelets implementing the functions of three platelet membrane proteins with different adhesive characteristics. J Atheroscler Thromb 22:201–210. https://doi.org/10.5551/jat.26203
Tosenberger A, Ataullakhanov F, Bessonov N, Panteleev M, Tokarev A, Volpert V (2012) Modelling of thrombus growth and growth stop in flow by the method of dissipative particle dynamics. Russ J Numer Anal Math Model 27:507–522. https://doi.org/10.1515/rnam-2012-0029
Tosenberger A, Ataullakhanov F, Bessonov N, Panteleev M, Tokarev A, Volpert V (2016) Modelling of platelet–fibrin clot formation in flow with a DPD-PDE method. J Math Biol 72:649–681. https://doi.org/10.1007/s00285-015-0891-2
Turrito VT, Baumgartner HR (1975) Platelet deposition on subendothelium exposed to flowing blood: mathematical analysis of physical parameters. Trans Am Soc Artif Intern Organs 21:593–601
Wang L, Zhang R, Zhang X, Hao P (2017) Numerical simulation of droplet impact on textured surfaces in a hybrid state. Microfluid Nanofluid 21:61. https://doi.org/10.1007/s10404-017-1900-0
Wootton DM, Markou CP, Hanson SR, Ku DN (2001) A mechanistic model of acute platelet accumulation in thrombogenic stenoses. Ann Biomed Eng 29:321–329
Wu W, Yang F, Wu J, Aubry N, Massoudi M, Antaki J (2016) High fidelity computational simulation of thrombus formation in Thoratec HeartMate II continuous flow ventricular assist device. Sci Rep. https://doi.org/10.1038/srep38025
Xu Z, Chen N, Kamocka MM, Rosen ED, Alber M (2008) A multiscale model of thrombus development. J R Soc Interface 5:705–722. https://doi.org/10.1098/rsif.2007.1202
Xu S, Xu Z, Kim OV, Litvinov RI, Weisel JW, Alber M (2017) Model predictions of deformation, embolization and permeability of partially obstructive blood clots under variable shear flow. J R Soc Interface 14:20170441. https://doi.org/10.1098/rsif.2017.0441
Yazdani A, Li H, Humphrey JD, Karniadakis GE (2017) A general shear-dependent model for thrombus formation. PLoS Comput Biol 13:e1005291. https://doi.org/10.1371/journal.pcbi.1005291
Zhang P, Gao C, Zhang N, Slepian MJ, Deng Y, Bluestein D (2014) Multiscale particle-based modeling of flowing platelets in blood plasma using dissipative particle dynamics and coarse grained molecular dynamics. Cell Mol Bioeng 7:552–574. https://doi.org/10.1007/s12195-014-0356-5
Acknowledgements
Research reported in this publication was partially supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (Award Nos. R01HL124170, R01HL131750). Liwei Wang was supported by the National Key Research and Development Program of China (Award Nos. 2017YFC0111100, 2016YFC1100300), National Natural Science Foundation of China (Award No. 11972215), and a Tsinghua Scholarship for Overseas Graduate Studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest in the subject matter or materials discussed in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Chen, Z., Zhang, J. et al. Modeling Clot Formation of Shear-Injured Platelets in Flow by a Dissipative Particle Dynamics Method. Bull Math Biol 82, 83 (2020). https://doi.org/10.1007/s11538-020-00760-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-020-00760-9