Abstract
Under the threat of predation, a species of prey can evolve to its own extinction. Matsuda and Abrams (Theor Popul Biol 45:76–91, 1994a) found the earliest example of evolutionary suicide by demonstrating that the foraging effort of prey can evolve until its population dynamics cross a fold bifurcation, whereupon the prey crashes to extinction. We extend this model in three directions. First, we use critical function analysis to show that extinction cannot happen via increasing foraging effort. Second, we extend the model to non-equilibrium systems and demonstrate evolutionary suicide at a fold bifurcation of limit cycles. Third, we relax a crucial assumption of the original model. To find evolutionary suicide, Matsuda and Abrams assumed a generalist predator, whose population size is fixed independently of the focal prey. We embed the original model into a three-species community of the focal prey, the predator and an alternative prey that can support the predator also alone, and investigate the effect of increasingly strong coupling between the focal prey and the predator’s population dynamics. Our three-species model exhibits (1) evolutionary suicide via a subcritical Hopf bifurcation and (2) indirect evolutionary suicide, where the evolution of the focal prey first makes the community open to the invasion of the alternative prey, which in turn makes evolutionary suicide of the focal prey possible. These new phenomena highlight the importance of studying evolution in a broader community context.




Similar content being viewed by others
References
Abrams PA, Harada Y, Matsuda H (1993) On the relationship between quantitative genetic and ESS models. Evolution 47:982–985
Bazykin AD (1998) Nonlinear dynamics of interacting populations. World Scientific Publishing Co., Singapore (edited by A. I. Khibnik and B. Krauskopf)
Berec L, Bernhauerová V, Boldin B (2018) Evolution of mate-finding Allee effect in prey. J Theor Biol 441:9–18
Boldin B, Kisdi E (2016) Evolutionary suicide through a non-catastrophic bifurcation: adaptive dynamics of pathogens with frequency-dependent transmission. J Math Biol 72:1101–1124
Boots M, Sasaki A (2003) Parasite evolution and extinctions. Ecol Lett 6:176–182
Bowers RG, Hoyle A, White A, Boots M (2005) The geometric theory of adaptive evolution: trade-off and invasion plots. J Theor Biol 233:363–377
de Mazancourt C, Dieckmann U (2004) Trade-off geometries and frequency-dependent selection. Am Nat 164:765–778
Dercole F, Ferriere R, Rinaldi S (2002) Ecological bistability and evolutionary reversals under asymmetrical competition. Evolution 56:1081–1090
Dercole F, Geritz SAH (2016) Unfolding the resident-invader dynamics of similar strategies. J Theor Biol 394:231–254
Dercole F, Prieu C, Rinaldi S (2010) Technological change and fisheries sustainability: the point of view of adaptive dynamics. Ecol Model 221:379–387
Dercole F, Rinaldi S (2008) Analysis of evolutionary processes. The adaptive dynamics approach and its applications. Princeton University Press, Princeton
Dieckmann U, Law R (1996) The dynamical theory of coevolution: a derivation from stochastic ecological processes. J Math Biol 34:579–612
Dhooge A, Govaerts W, Kuznetsov YA, Meijer HGE, Sautois B (2008) New features of the software MatCont for bifurcation analysis of dynamical systems. Math Comput Model Dyn Syst 14:147–175
Geritz SAH (2005) Resident-invader dynamics and the coexistence of similar strategies. J Math Biol 50:67–82
Geritz SAH, Gyllenberg M (2012) A mechanistic derivation of the DeAngelis–Beddington functional response. J Theor Biol 314:106–108
Geritz SAH, Gyllenberg M (2014) The DeAngelis–Beddington functional response and the evolution of timidity of the prey. J Theor Biol 359:37–44
Geritz SAH, Kisdi E, Meszéna G, Metz JAJ (1998) Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol Ecol 12:35–57
Geritz SAH, Gyllenberg M, Jacobs FJA, Parvinen K (2002) Invasion dynamics and attractor inheritance. J Math Biol 44:548–560
Geritz SAH, Kisdi E, Yan P (2007) Evolutionary branching and long-term coexistence of cycling predators: critical function analysis. Theor Popul Biol 71:424–435
Groll F, Arndt H, Altland A (2017) Chaotic attractor in two-prey one-predator system originates from interplay of limit cycles. Theor Ecol 10:147–154
Gyllenberg M (2008) Evolutionary suicide. ERCIM News 73:18
Gyllenberg M, Parvinen K (2001) Necessary and sufficient conditions for evolutionary suicide. Bull Math Biol 63:981–993
Gyllenberg M, Parvinen K, Dieckmann U (2002) Evolutionary suicide and evolution of dispersal in structured metapopulations. J Math Biol 45:79–105
Hin V, de Roos AM (nd) Cannibalism prevents evolutionary suicide of ontogenetic omnivores in life history intraguild predation systems. Manuscript in preparation
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398
Holt RD (1977) Predation, apparent competition, and the structure of prey communities. Theor Popul Biol 12:197–229
Kisdi E (2006) Trade-off geometries and the adaptive dynamics of two coevolving species. Evol Ecol Res 8:959–973
Kisdi E (2015) Construction of multiple trade-offs to obtain arbitrary singularities of adaptive dynamics. J Math Biol 70:1093–1117
Kisdi E, Geritz SAH, Boldin B (2013) Evolution of pathogen virulence under selective predation: a construction method to find eco-evolutionary cycles. J Theor Biol 339:140–150
Krivan V, Eisner J (2006) The effect of the Holling type II functional response on apparent competition. Theor Popul Biol 70:421–430
Kuznetsov YA (1998) Elements of applied bifurcation theory, 2nd edn. Springer, New York
Matsuda H, Abrams PA (1994a) Timid consumers: self-extinction due to adaptive change in foraging and anti-predator effort. Theor Popul Biol 45:76–91
Matsuda H, Abrams PA (1994b) Runaway evolution to self-extinction under asymmetrical competition. Evolution 48:1764–1772
Maynard Smith J (1982) Evolution and the theory of games. Cambridge University Press, Cambridge
Parvinen K, Dieckmann U (2013) Self-extinction through optimizing selection. J Theor Biol 333:1–9
Rinaldi S, Muratori S (1992) Slow-fast limit cycles in predator–prey models. Ecol Model 61:287–308
terHorst CP, Zee PC, Heath KD, Miller TE, Pastore AI, Patel S, Schreiber SJ, Wade MJ, Walsh MR (2018) Evolution in a community context: trait responses to multiple species interactions. Am Nat 191:368–380
Thieme H (2003) Mathematics in population biology. Princeton University Press, Princeton
Van Voorn GAK, Hemerik L, Boer MP, Kooi BW (2007) Heteroclinic orbits indicate overexploitation in predator–prey systems with a strong Allee effect. Math Biosci 209:451–469
Vitale C (2016) Evolutionary suicide in a two-prey-one-predator model with Holling type II functional response. MSc thesis, University of Helsinki. http://hdl.handle.net/10138/163028. Accessed 7 Aug 2018
Walsh MR (2013) The evolutionary consequences of indirect effects. Trends Ecol Evol 28:23–29
Webb C (2003) A complete classification of Darwinian extinction in ecological interactions. Am Nat 161:181–205
Acknowledgements
We thank Francesca Scarabel for help with MatCont, and anonymous reviewers for their comments on an earlier version of this paper. C.V. was partially supported by a Leverhulme Trust studentship as part of the Centre for Applied Biological Modelling at the University of Sheffield. E.K. was funded by the Academy of Finland through the Centre of Excellence in Analysis and Dynamics.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
In this appendix, we investigate the interior equilibria \((\bar{n}_1,\bar{n}_2,\bar{p})\) of the population dynamics in Eq. (4). Using the notation
for abbreviating the density-independent part of the per capita growth rates as well as
for the Holling II factors, the equilibrium equations (assuming nonzero population densities \(\bar{n}_1\), \(\bar{n}_2\), \(\bar{p}\)) are
First, we express \(\bar{n}_1\) and \(\bar{n}_2\) from (12),
Next, we eliminate \(\bar{p}\) by dividing (13a) with (13b), and substitute \(\bar{n}_1\) and \(\bar{n}_2\) with the above expressions to rewrite the equation in terms of \(\phi _1\) and \(\phi _2\),
which is rearranged into
Substituting \(\phi _2=(\mu -\alpha _1 \phi _1)/\alpha _2\) from (13c), we obtain a cubic polynomial for \(\phi _1\). Each root of this cubic equation yields a root of the equilibrium equations in Eq. (13) with \(\phi _2\) from (13c), \(\bar{n}_1\) and \(\bar{n}_2\) from (14), and \(\bar{p}\) from (13a). Figure 29 of Vitale (2016) shows an example where all three roots are real and positive, i.e., biologically admissible equilibria of the model.
If \(\alpha _1=0\), then \(\phi _2=\mu /\alpha _2\) is a constant independent of \(\phi _1\). Therefore, (15) is only quadratic in \(\phi _1\), yielding at most two interior equilibria.
Appendix B
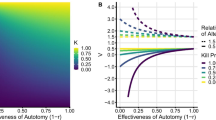
In Sect. 3, we show that evolutionary suicide occurs at a given trait value \(c_1^*\) if the birth rate function at this point has a specific value \(B(c_1^*)\) given by (8) and a slope \(B'(c_1^*)\) in the interval given by inequalities (9) and (10). The figure below shows the width of this interval,
Evolutionary suicide is impossible if this width is negative (gray area). The width depends on the chosen value of \(c_1^*\) [panel (a)]. It is easy to see also analytically that the width increases with \(q \bar{p}\) [all panels] and with \(\beta _1\) [panel (b)], and has a maximum as a function of the composite parameter \((c_1^*)^2 (h_1/\delta _1)\) [panels (a), (c) of Fig. 5].
The width of the interval for \(B'(c_1^*)\) that results in evolutionary suicide. The contour lines, starting from the bottom line, correspond to values \(0, 0.1, 0.2, \ldots , 0.7\). In the gray area (i.e., below the 0 contour line), condition (7) is violated and hence evolutionary suicide is not possible. Parameter values: a\(h_1/\delta _1 = 4, \beta _1 = 1\); b\(h_1/\delta _1 = 4, c_1^*=1.2\); c\(\beta _1 = 1, c_1^*=1.2\)
Appendix C
Here, we consider a well-mixed system where all species are present in one habitat and the predator searches for both prey simultaneously all time. This alternative model is given by
where \(B_1\) and \(B_2\) abbreviate the birth rates of prey species 1 and 2, respectively. Note that in contrast to Eq. (4), here both prey densities appear in the denominator of the Holling II terms. To interpret this difference, recall that 1 over the denominator of the Holling II functional response gives the fraction of predators that are searching (as opposed to handling an already captured prey). In the model of (16), handling any of the prey in the well-mixed system removes a predator individual from the searching predators. In our main model (4), in a fraction q of its time a predator can only capture, and therefore handle, prey species 1, whereas in the remaining \(1-q\) fraction of time it can handle only species 2. Thus, within each time frame, only handling one prey species removes an individual from the searching predators; accordingly, only one prey species appears in the functional response in Eq. (4).
The model in (16) naturally extends to k prey species,
To find the equilibria, let S denote the number of searching predators,
At an interior equilibrium of (17), we have
This is a linear system for the unknowns \(n_1,\ldots ,n_k,S\), and hence generically has only one solution for the equilibrium. The equilibrium value of p follows directly from (18). Since the population dynamics in (16) has a unique interior equilibrium, it cannot undergo a saddle-node bifurcation for any change of the traits such as the foraging effort \(c_1\) and the birth rate \(B_1\).
The four-species model
unites the features of our main model in Eq. (4) and the alternative model in (16); here, the focal prey 1 and prey 3 live in the first habitat where the predator spends a fraction q of its time, and the alternative prey 2 lives in the second habitat where the predator spends the remaining fraction \((1-q)\) of its time. This model is briefly mentioned in Discussion.
Rights and permissions
About this article
Cite this article
Vitale, C., Kisdi, E. Evolutionary Suicide of Prey: Matsuda and Abrams’ Model Revisited. Bull Math Biol 81, 4778–4802 (2019). https://doi.org/10.1007/s11538-018-0472-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-0472-9
Keywords
- Adaptive dynamics
- Evolutionary suicide
- Fold bifurcation of limit cycles
- Foraging effort
- Predator–prey model
- Saddle-node bifurcation
- Subcritical Hopf bifurcation