Abstract
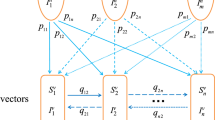
A class of models that describes the interactions between multiple host species and an arthropod vector is formulated and its dynamics investigated. A host-vector disease model where the host’s infection is structured into n stages is formulated and a complete global dynamics analysis is provided. The basic reproduction number acts as a sharp threshold, that is, the disease-free equilibrium is globally asymptotically stable (GAS) whenever \({\mathcal {R}}_0^2\le 1\) and that a unique interior endemic equilibrium exists and is GAS if \({\mathcal {R}}_0^2>1\). We proceed to extend this model with m host species, capturing a class of zoonoses where the cross-species bridge is an arthropod vector. The basic reproduction number of the multi-host-vector, \({\mathcal {R}}_0^2(m)\), is derived and shown to be the sum of basic reproduction numbers of the model when each host is isolated with an arthropod vector. It is shown that the disease will persist in all hosts as long as it persists in one host. Moreover, the overall basic reproduction number increases with respect to the host and that bringing the basic reproduction number of each isolated host below unity in each host is not sufficient to eradicate the disease in all hosts. This is a type of “amplification effect,” that is, for the considered vector-borne zoonoses, the increase in host diversity increases the basic reproduction number and therefore the disease burden.



Similar content being viewed by others
References
Anderson RM, May RM (1981) The population dynamics of microparasites and their invertebrate hosts. Philos Trans R Soc Lond B Biol Sci 291:451–524
Anderson RM, May RM (1991) Infectious diseases of humans, dynamics and control. Oxford Science Publications, Oxford
Begon M, Bowers RG (1994) Host–host–pathogen models and microbial pest control: the effect of host self regulation. J Theor Biol 169:275–287
Begon M, Bowers RG, Kadianakis N, Hodgkinson DE (1992) Disease and community structure: the importance of host self-regulation in a host–host–pathogen model. Am Nat 139:1131–1150
Bhatia NP, Szegö GP (1967) Dynamical systems: stability theory and applications. Lecture notes in mathematics, no. 35. Springer, Berlin
Bhatia NP, Szegö GP (1970) Stability theory of dynamical systems. Springer, Berlin
Bowers RG, Begon M (1991) A host–host–pathogen model with free-living infective stages, applicable to microbial pest control. J Theor Biol 148:305–329
Bowman C, Gumel A, van den Driessche P, Wu J, Zhu H (2005) A mathematical model for assessing control strategies against West Nile virus. Bull Math Biol 67:1107–1133
Castillo-Chavez C, Thieme HR (1995) Asymptotically autonomous epidemic models. In: Arino O, D.E A, Kimmel M (eds) Mathematical population dynamics: analysis of heterogeneity. Theory of epidemics, vol 1. Wuerz, Winnipeg
Centers for Disease Control and Prevention (2015a) Tickborne diseases of the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/lyme/resources/tickbornediseases.pdf. Accessed 20 May 2017
Centers for Disease Control and Prevention (2015b) Transmission of Japanese encephalitis virus. Centers for Disease Control and Prevention. https://www.cdc.gov/japaneseencephalitis/symptoms/index.html. Accessed 20 May 2017
Centers for Disease Control and Prevention (2015c) Zoonotic diseases. Centers for Disease Control and Prevention. https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html. Accessed 20 May 2017
Centers for Disease Control and Prevention (2017) One health. Centers for Disease Control and Prevention. https://www.cdc.gov/onehealth/. Accessed 20 May 2017
Cruz-Pacheco G, Esteva L, Vargas C (2012a) Control measures for chagas disease. Math Biosci 237:49–60
Cruz-Pacheco G, Esteva L, Vargas C (2012b) Multi-species interactions in West Nile virus infection. J Biol Dyn 6:281–298
Diekmann O, Heesterbeek JAP (2000) Mathematical epidemiology of infectious diseases: model building, analysis and interpretation. Wiley series in mathematical and computational biology. Wiley, Chichester
Dobson A (2004) Population dynamics of pathogens with multiple host species. Am Nat 164:S64–S78
Dworkin MS, Schwan TG, Anderson DE, Borchardt SM (2008) Tick-borne relapsing fever. Infect Dis Clin N Am 22:449–468
Franco JR, Simarro PP, Diarra A, Jannin JG (2014) Epidemiology of human African trypanosomiasis. Clin Epidemiol 6:257–275
Greenman J, Hudson P (2000) Parasite-mediated and direct competition in a two-host shared macroparasite system. Theor Popul Biol 57:13–34
Guo H, Li M (2006a) Global dynamics of a staged progression model for infectious diseases. Math Biosci Eng 3:513–525
Guo H, Li MY (2006b) Global dynamics of a staged progression model for infectious diseases. Math Biosci Eng 3:513
Guo H, Li MY, Shuai Z (2012) Global dynamics of a general class of multistage models for infectious diseases. SIAM J Appl Math 72:261–279
Holt RD, Pickering J (1985) Infectious disease and species coexistence: a model of Lotka–Volterra form. Am Nat 126:196–211
Homer MJ, Aguilar-Delfin I, Telford SR, Krause PJ, Persing DH (2000) Babesiosis. Clin Microbiol Rev 13:451–469
Iggidr A, Mbang J, Sallet G, Tewa J (2007) Multi-compartment models. DCDS Suppl 506–519
Johnson CK, Hitchens PL, Evans TS, Goldstein T, Thomas K, Clements A, Joly DO, Wolfe ND, Daszak P, Karesh WB, Mazet JK (2015) Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci Rep 5:14830
Johnson TL, Landguth EL, Stone EF (2016) Modeling relapsing disease dynamics in a host-vector community. PLoS Negl Trop Dis 10:e0004428
Kermack WO, McKendrick AG (1927) Contributions to the mathematical theory of epidemics-I. Bull Math Biol 53(1991):33–55
Khan SU, Salje H, Hannan A, Islam MA, Bhuyan AM, Islam MA, Rahman MZ, Nahar N, Hossain MJ, Luby SP et al (2014) Dynamics of Japanese encephalitis virus transmission among pigs in Northwest Bangladesh and the potential impact of pig vaccination. PLoS Negl Trop Dis 8:e3166
LaSalle J (1968) Stability theory for ordinary differential equations. J Differ Equ 4:57–65
LaSalle JP, Lefschetz S (1961) Stability by Liapunov’s direct method. Academic Press, Cambridge
Lloyd AL, May RM (1996) Spatial heterogeneity in epidemic models. J Theor Biol 179:1–11
Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JR, Dobson AP, Hudson PJ, Grenfell BT (2009) Epidemic dynamics at the human–animal interface. Science 326:1362–1367
Marfin AA, Petersen LR, Eidson M, Miller J, Hadler J, Farello C, Werner B, Campbell GL, Layton M, Smith P et al (2001) Widespread west Nile virus activity, eastern United States, 2000. Emerg Infect Dis 7:730
McDonald JL, Bailey T, Delahay RJ, McDonald RA, Smith GC, Hodgson D (2016) Demographic buffering and compensatory recruitment promotes the persistence of disease in a wildlife population. Ecol Lett 19:443–449
Miller E, Huppert A (2013) The effects of host diversity on vector-borne disease: the conditions under which diversity will amplify or dilute the disease risk. PLoS ONE 8:e80279
One Health (2017) One health. One Health Commission. https://www.onehealthcommission.org/. Accessed 25 May 2017
Ostfeld RS, Keesing F (2012) Effects of host diversity on infectious disease. Annu Rev Ecol Evol Syst 43:157–182
Palmer C, Landguth E, Stone E, Johnson T (2016) The dynamics of vector-borne relapsing diseases. arXiv preprint arXiv:1605.07647
Park TR (2004) Age-dependence in epidemic models of vector-borne infections. PhD thesis, University of Alabama in Huntsville
Ross R (1911) The prevention of malaria. John Murray, London
Ross R (1916) An application of the theory of probabilities to the study of a priori pathometry. Part I. Proc R Soc Lond A 92:204–230
Ross R, Hudson H (1917a) An application of the theory of probabilities to the study of a priori pathometry. Part II. Proc R Soc Lond A 93:212–225
Ross R, Hudson H (1917b) An application of the theory of probabilities to the study of a priori pathometry. Part III. Proc R Soc Lond A 93:225–240
Salkeld DJ, Padgett KA, Jones JH (2013) A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol Lett 16:679–686
Simpson JE, Hurtado PJ, Medlock J, Molaei G, Andreadis TG, Galvani AP, Diuk-Wasser MA (2011) Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc R Soc Lond B Biol Sci rspb20111282
Southern PM Jr, Sanford JP (1969) Relapsing fever: a clinical and microbiological review. Medicine 48:129–150
Taylor LH, Latham SM, Mark E (2001) Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci 356:983–989
Thrall PH, Antonovics J, Hall DW (1993) Host and pathogen coexistence in sexually transmitted and vector-borne diseases characterized by frequency-dependent disease transmission. Am Nat 142:543–552
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
Velascohernandez JX (1994) A model for chagas disease involving transmission by vectors and blood transfusion. Theor Popul Biol 46:1–31
Vidyasagar M (1980) Decomposition techniques for large-scale systems with nonadditive interactions: stability and stabilizability. IEEE Trans Autom Control 25:773–779
Wengert O, Kopp M, Siebert E, Stenzel W, Hegasy G, Suttorp N, Stich A, Zoller T (2014) Human african trypanosomiasis with 7-year incubation period: clinical, laboratory and neuroimaging findings. Parasitol Int 63:557–560
World Bank (2010) People, pathogens and our planet: towards a one health approach for controlling zoonotic diseases, vol 1. World Bank, Agriculture and Rural Development Health, Nutrition and Population, Washington
Acknowledgements
We are grateful to two anonymous referees for valuable comments and suggestions that led to an improvement in this paper. A. Iggidr acknowledges the partial support of Inria in the framework of the program STIC AmSud (Project MOSTICAW).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bichara, D., Iggidr, A. & Smith, L. Multi-stage Vector-Borne Zoonoses Models: A Global Analysis. Bull Math Biol 80, 1810–1848 (2018). https://doi.org/10.1007/s11538-018-0435-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-0435-1
Keywords
- Vector-borne zoonoses
- Stage progression
- Multi-host
- One health
- Amplification effect
- Global stability
- Nonlinear dynamical systems