Abstract
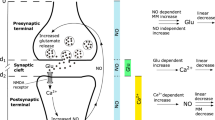
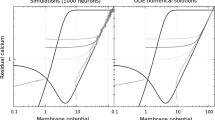
Information in the brain is stored in a form of an altered synaptic strength between neurons. The long-term potentiation (LTP), a phenomenon when a short-term increase in neural activity is transformed into a long-lasting increase in strengths of synaptic connectivity, provides an experimental substrate of memory. Using reaction–diffusion equations, we established an LTP model, describing the dynamics of glutamate (Glu), calcium (Ca2+) and nitric oxide (NO) in response to the stimulus—a presynaptic action potential. NO can diffuse to the presynaptic terminal and facilitate the Glu release forming a positive feedback loop. Therefore, the LTP can be considered as a chain of biochemical reactions with a positive feedback loop. In this study, we investigated numerically the role of interactions in a chain of biochemical reactions with a positive feedback on the bistable behavior or memory. We conclude that the positive feedback system with the linear interaction between substances does not exhibit a bistable behavior. However, introduction of substrate saturation described by Michaelis–Menten kinetics for NO decay can lead to an increase in synaptic strength lasting for dozens or even hundreds of seconds. Our finding extends a possible role of NO in LTP: a short high intensity stimulus is “memorized” as a long-lasting elevation of NO concentration.






Similar content being viewed by others
References
Baronas R, Ivanauskas F, Kulys J (2010) The difference schemes for the reaction–diffusion equations. Mathematical modeling of biosensors. Springer, Dordrecht, pp 293–315
Blitzer RD (2005) Long-term potentiation: mechanisms of induction and maintenance. Sci Signal 2005:tr26
Bon CL, Garthwaite J (2001) Nitric oxide-induced potentiation of CA1 hippocampal synaptic transmission during baseline stimulation is strictly frequency-dependent. Neuropharmacology 40:501–507
Bradley SA, Steinert JR (2016) Nitric oxide-mediated posttranslational modifications: impacts at the synapse. Oxid Med Cell Longev 2016:5681036
Bronner F (2001) Extracellular and intracellular regulation of calcium homeostasis. Sci World J 1:919–925
Castillo PE (2012) Presynaptic LTP and LTD of excitatory and inhibitory synapses. Cold Spring Harb Perspect Biol 4(2):a005728
Chater TE, Goda Y (2014) The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front Cell Neurosci 8:401
Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL (1992) The time course of glutamate in the synaptic cleft. Science 258:1498–1501
Frade JG, Barbosa RM, Laranjinha J (2009) Stimulation of NMDA and AMPA glutamate receptors elicits distinct concentration dynamics of nitric oxide in rat hippocampal slices. Hippocampus 19:603–611
Garthwaite J (2008) Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci 27:2783–2802
Garthwaite J (2016) From synaptically localized to volume transmission by nitric oxide. J Physiol 594:9–18
Garthwaite J, Charles SL, Chess-Williams R (1988) Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336:385–388
Haley JE, Wilcox GL, Chapman PF (1992) The role of nitric oxide in hippocampal long-term potentiation. Neuron 8:211–216
Hall CN, Garthwaite J (2009) What is the real physiological NO concentration in vivo? Nitric Oxide 21:92–103
Hardingham N, Dachtler J, Fox K (2013) The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front Cell Neurosci 7:190
Herring BE, Nicoll RA (2016) Long-term potentiation: from CaMKII to AMPA receptor trafficking. Annu Rev Physiol 78:351–365
Hopper RA, Garthwaite J (2006) Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J Neurosci 26:11513–11521
Huang EP (1997) Synaptic plasticity: a role for nitric oxide in LTP. Curr Biol 7:R141–R143
Izaki Y, Takita M, Nomura M, Akema T (2003) Differences between paired-pulse facilitation and long-term potentiation in the dorsal and ventral hippocampal CA1-prefrontal pathways of rats. Brain Res 992:142–145
Kim HY, Jun J, Wang J, Bittar A, Chung K, Chung JM (2015) Induction of long-term potentiation and long-term depression is cell-type specific in the spinal cord. Pain 156:618–625
Kovalchuk Y, Eilers J, Lisman J, Konnerth A (2000) NMDA receptor-mediated subthreshold Ca2+ signals in spines of hippocampal neurons. J Neurosci 20:1791–1799
Laranjinha J, Santos RM, Lourenço CF, Ledo A, Barbosa RM (2012) Nitric oxide signaling in the brain: translation of dynamics into respiration control and neurovascular coupling. Ann NY Acad Sci 1259:10–18
Lomo T (1966) Frequency potentiation of excitatory synaptic activity in the dentate area of the hippocampal formation. Acta Physiol Scand 68(277):128
Lüscher C, Malenka RC (2012) NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol 4(6):a005710
Manninen T, Hituri K, Kotaleski JH, Blackwell KT, Linne ML (2010) Postsynaptic signal transduction models for long-term potentiation and depression. Front Comput Neurosci 4:152
Mayford M, Siegelbaum SA, Kandel ER (2012) Synapses and memory storage. Cold Spring Harb Perspect Biol 4(6):a005751
Mazzoni A, Broccard FD, Garcia-Perez E, Bonifazi P, Ruaro ME, Torre V (2007) On the dynamics of the spontaneous activity in neuronal networks. PLoS ONE 2:e439
McCormick DA (2005) Neuronal networks: flip-flops in the brain. Curr Biol 15:R294–R296
Mincheva M, Craciun G (2008) Multigraph conditions for multistability, oscillations and pattern formation in biochemical reaction networks. Proc IEEE 96:1281–1291
Muñoz FJ, Godoy JA, Cerpa W, Poblete IM, Huidobro-Toro JP, Inestrosa NC (2014) Wnt-5a increases NO and modulates NMDA receptor in rat hippocampal neurons. Biochem Biophys Res Commun 444:189–194
Neitz A, Mergia E, Imbrosci B, Petrasch-Parwez E, Eysel UT, Koesling D, Mittmann T (2014) Postsynaptic NO/cGMP increases NMDA receptor currents via hyperpolarization-activated cyclic nucleotide-gated channels in the hippocampus. Cereb Cortex 24(7):1923–1936
Parodi J, Montecinos-Oliva C, Varas R, Alfaro IE, Serrano FG, Varas-Godoy M, Muñoz FJ, Cerpa W, Godoy JA, Inestrosa NC (2015) Wnt5a inhibits K(+) currents in hippocampal synapses through nitric oxide production. Mol Cell Neurosci 68:314–322
Penn Y, Segal M, Moses E (2016) Network synchronization in hippocampal neurons. Proc Natl Acad Sci USA 113:3341–3346
Pigott BM, Garthwaite J (2016) Nitric oxide is required for L-Type Ca(2+) channel-dependent long-term potentiation in the hippocampus. Front Synaptic Neurosci 8:17
Prinz AA, Bucher D, Marder E (2004) Similar network activity from disparate circuit parameters. Nat Neurosci 7(12):1345–1352
Sala F, Hernández-Cruz A (1990) Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J 57:313–324
Samarskii AA (2001) The theory of difference schemes. CRC Press, New York
Susswein AJ, Katzoff A, Miller N, Hurwitz I (2004) Nitric oxide and memory. The Neuroscientist 10:153–162
Ventriglia F, Di Maio V (2000) A Brownian model of glutamate diffusion in excitatory synapses of hippocampus. Biosystems 58:67–74
Volgushev M, Balaban P, Chistiakova M, Eysel UT (2000) Retrograde signalling with nitric oxide at neocortical synapses. Eur J Neurosci 12:4255–4267
Wang Q, Mergia E, Koesling D, Mittmann T (2017) Nitric oxide/cGMP signaling via guanylyl cyclase isoform 1 modulates glutamate and GABA release in somatosensory cortex of mice. Neuroscience 360:180–189
Zacharia IG, Deen WM (2005) Diffusivity and solubility of nitric oxide in water and saline. Ann Biomed Eng 33:214–222
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katauskis, P., Ivanauskas, F. & Alaburda, A. The “Memory” Effect in a Chain of Biochemical Reactions with a Positive Feedback is Enhanced by Substrate Saturation Described by Michaelis–Menten Kinetics. Bull Math Biol 81, 919–935 (2019). https://doi.org/10.1007/s11538-018-00541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-00541-5