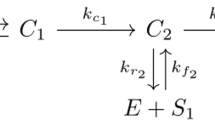Abstract
There are many intracellular signalling pathways where the spatial distribution of the molecular species cannot be neglected. These pathways often contain negative feedback loops and can exhibit oscillatory dynamics in space and time. Two such pathways are those involving Hes1 and p53–Mdm2, both of which are implicated in cancer.
In this paper we further develop the partial differential equation (PDE) models of Sturrock et al. (J. Theor. Biol., 273:15–31, 2011) which were used to study these dynamics. We extend these PDE models by including a nuclear membrane and active transport, assuming that proteins are convected in the cytoplasm towards the nucleus in order to model transport along microtubules. We also account for Mdm2 inhibition of p53 transcriptional activity.
Through numerical simulations we find ranges of values for the model parameters such that sustained oscillatory dynamics occur, consistent with available experimental measurements. We also find that our model extensions act to broaden the parameter ranges that yield oscillations. Hence oscillatory behaviour is made more robust by the inclusion of both the nuclear membrane and active transport. In order to bridge the gap between in vivo and in silico experiments, we investigate more realistic cell geometries by using an imported image of a real cell as our computational domain. For the extended p53–Mdm2 model, we consider the effect of microtubule-disrupting drugs and proteasome inhibitor drugs, obtaining results that are in agreement with experimental studies.























Similar content being viewed by others
References
Agrawal, S., Archer, C., & Schaffer, D. (2009). Computational models of the notch network elucidate mechanisms of context-dependent signaling. PLoS Comput. Biol., 5, e1000390.
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2008). Molecular biology of the cell. Garland science (5th ed.). Oxford: Taylor and Francis.
Bancaud, A., Huet, S., Daigle, N., Mozziconacci, J., Beaudouin, J., & Ellenberg, J. (2009). Molecular crowding affects diffusion and binding of nuclear proteins in heterochromatin and reveals the fractal organization of chromatin. EMBO J., 28, 3785–3798.
Barik, D., Baumann, W. T., Paul, M. R., Novak, B., & Tyson, J. J. (2010). A model of yeast cell-cycle regulation based on multisite phosphorylation. Mol. Syst. Biol., 6, 405.
Barik, D., Paul, M. R., Baumann, W. T., Cao, Y., & Tyson, J. J. (2008). Stochastic simulation of enzyme-catalyzed reactions with disparate timescales. Biophys. J., 95, 3563–3574.
Barrio, M., Burrage, K., Leier, A., & Tian, T. (2006). Oscillatory regulation of Hes1: discrete stochastic delay modelling and simulation. PLoS ONE, 2, e117.
Baserga, R. (2007). Is cell size important? Cell Cycle, 6(7), 814–816.
Batchelor, E., Loewer, A., & Lahav, G. (2009). The ups and downs of p53: understanding protein dynamics in single cells. Nat. Rev., Cancer, 9(5), 371–377.
Batchelor, E., Mock, C., Bhan, I., Loewer, A., & Lahav, G. (2008). Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol. Cell, 30, 277–289.
Beck, M., Forster, F., Ecke, M., Plitzko, J. M., Melchoir, F., Gerisch, G., Baumeister, W., & Medalia, O. (2004). Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science, 306, 1387–1390.
Bernard, S., Čajavec, B., Pujo-Menjouet, L., Mackey, M., & Herzel, H. (2006). Modelling transcriptional feedback loops: the role of Gro/TLE1 in Hes1 oscillations. Philos. Trans. R. Soc. A, 364, 1155–1170.
Brown, G., & Kholodenko, B. (1999). Spatial gradients of cellular phospho-proteins. FEBS Lett., 457, 452–454.
Busenberg, S., & Mahaffy, J. (1985). Interaction of spatial diffusion and delays in models of genetic control by repression. J. Math. Biol., 22, 313–333.
Cangiani, A., & Natalini, R. (2010). A spatial model of cellular molecular trafficking including active transport along microtubules. J. Theor. Biol., 267, 614–625.
Carbonaro, M., O’Brate, A., & Giannakakou, P. (2011). Microtubule disruption targets HIF-1α mRNA to cytoplasmic P-bodies for translation repression. J. Cell Biol., 192, 83–99.
Caspi, A., Granek, R., & Elbaum, M. (2000). Enhanced diffusion in active intracellular transport. Phys. Rev. Lett., 85, 5655–5658.
Chahine, M. N., & Pierce, G. N. (2009). Therapeutic targeting of nuclear protein import in pathological cell conditions. Pharmacol. Rev., 61, 358–372.
Ciliberto, A., Novak, B., & Tyson, J. (2005). Steady states and oscillations in the p53/Mdm2 network. Cell Cycle, 4, 488–493.
Cole, C., & Scarcelli, J. (2006). Transport of messenger RNA from the nucleus to the cytoplasm. Curr. Opin. Cell Biol., 18, 299–306.
Cole, N., & Lippincott-Schwartz, J. (1995). Organization of organelles and membrane traffic by microtubules. Curr. Opin. Cell Biol., 7, 55–64.
Davidson, M. W. (2011). Micromagnet website. http://micro.magnet.fsu.edu/primer/techniques/fluorescence/gallery/cells/u2/u2cellslarge8.html (accessed 26th April 2011)
Diller, L., Kassel, J., Nelson, C., Gryka, M., Litwak, G., Gebhardt, M., Bressac, B., Ozturk, M., Baker, S., Vogelstein, B., & Friend, S. (1990). p53 functions as a cell cycle control protein in osteosarcomas. Mol. Cell. Biol., 10, 5772–5781.
Dinh, A., Theofanous, T., & Mitragotri, S. (2005). A model of intracellular trafficking of adenoviral vectors. Biophys. J., 89, 1574–1588.
Feldherr, C., & Akin, D. (1991). Signal-mediated nuclear transport in proliferating and growth-arrested BALB/c 3T3 cells. J. Cell Biol., 115, 933–939.
Ferrari, S., & Palmerini, E. (2007). Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr. Opin. Oncol., 19, 341–346.
Finlay, C. (1993). The mdm-2 oncogene can overcome wild-type p53 suppression of transformed cell growth. Mol. Cell. Biol., 12, 301–306.
Gasiorowski, J. Z., & Dean, D. (2003). Mechanisms of nuclear transport and interventions. Adv. Drug Deliv. Rev., 55, 703–716.
Geva-Zatorsky, N., Rosenfeld, N., Itzkovitz, S., Milo, R., Sigal, A., Dekel, E., Yarnitzky, T., Liron, Y., Polak, P., Lahav, G., & Alon, U. (2006). Oscillations and variability in the p53 system. Mol. Syst. Biol., 2, E1–E13.
Giannakakou, P., Sackett, D., Ward, Y., Webster, K., Blagosklonny, M., & Fojo, T. (2000). p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat. Cell Biol., 2(10), 709–717.
Glass, L., & Kauffman, S. (1972). Co-operative components, spatial localization and oscillatory cellular dynamics. J. Theor. Biol., 34, 219–237.
Gordon, K., Leeuwen, I. V., Laín, S., & Chaplain, M. (2009). Spatio-temporal modelling of the p53–mdm2 oscillatory system. Math. Model. Nat. Phenom., 4, 97–116.
Hamstra, D., Bhojani, M., Griffin, L., Laxman, B., Ross, B., & Rehemtulla, A. (2006). Real-time evaluation of p53 oscillatory behaviour in vivo using bioluminescent imaging. Cancer Res., 66, 7482–7489.
Hirata, H., Yoshiura, S., Ohtsuka, T., Bessho, Y., Harada, T., Yoshikawa, K., & Kageyama, R. (2002). Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science, 298, 840–843.
Johansson, T., Lejonklou, M., Ekeblad, S., Stålberg, P., & Skogseid, B. (2008). Lack of nuclear expression of hairy and enhancer of split-1 (HES1) in pancreatic endocrine tumors. Horm. Metab. Res., 40, 354–359.
Jordan, M. A., & Wilson, L. (2004). Microtubules as a target for anticancer drugs. Nat. Rev., Cancer, 4, 253–265.
Kar, S., Baumann, W. T., Paul, M. R., & Tyson, J. J. (2009). Exploring the roles of noise in the eukaryotic cell cycle. Proc. Natl. Acad. Sci. USA, 106, 6471–6476.
Kau, T., Way, J., & Silver, P. (2004). Nuclear transport and cancer: from mechanism to intervention. Nature, 4, 106–117.
Kavallaris, M. (2010). Microtubules and resistance to tubulin-binding agents. Nat. Rev., Cancer, 10, 194–204.
Kherlopian, A., Song, T., Duan, Q., Neimark, M., Po, M., Gohagan, J., & Laine, A. (2008). A review of imaging techniques for systems biology. BMC Syst. Biol., 2, 74–92.
Kholodenko, B. (2006). Cell-signalling dynamics in time and space. Nat. Rev. Mol. Cell Biol., 7, 165–174.
Kim, I., Kim, D., Han, S., Chin, M., Nam, H., Cho, H., Choi, S., Song, B., Kim, E., Bae, Y., & Moon, Y. (2000). Truncated form of importin alpha identified in breast cancer cells inhibits nuclear import of p53. J. Biol. Chem., 275, 23139–23145.
Klonis, N., Rug, M., Harper, I., Wickham, M., Cowman, A., & Tilley, L. (2002). Fluorescence photobleaching analysis for the study of cellular dynamics. Eur. Biophys. J., 31, 36–51.
Lahav, G., Rosenfeld, N., Sigal, A., Geva-Zatorsky, N., Levine, A., Elowitz, M., & Alon, U. (2004). Dynamics of the p53–Mdm2 feedback loop in individual cells. Nat. Genet., 36, 147–150.
Lane, D. (1992). p53, guardian of the genome. Nature, 358, 15–16.
Lewis, J. (2003). Autoinhibition with transcriptional delay: a simple mechanism for the zebrafish somitogenesis oscillator. Curr. Biol., 13, 1398–1408.
Lightcap, E., McCormack, T., Pien, C., Chau, V., Adams, J., & Elliott, P. (2000). Proteasome inhibition measurements: clinical application. Clin. Chem., 46, 673–683.
Locke, J., Southern, M., Kozma-Bognár, L., Hibberd, V., Brown, P., Turner, M., & Mllar, A. (2005). Extension of a genetic network model by iterative experimentation and mathematical analysis. Mol. Syst. Biol., 1, 1–9.
Loewer, A., Batchelor, E., Gaglia, G., & Lahav, G. (2010). Basal dynamics of p53 reveal transcriptionally attenuated pulses in cycling cells. Cell, 142(1), 89–100.
Lomakin, A., & Nadezhdina, E. (2010). Dynamics of nonmembranous cell components: role of active transport along microtubules. Biochemistry (Moscow), 75(1), 7–18.
Ma, L., Wagner, J., Rice, J., Hu, W., Levine, A., & Stolovitzky, G. (2005). A plausible model for the digital response of p53 to DNA damage. Proc. Natl. Acad. Sci. USA, 102, 14266–14271.
Mahaffy, J. (1988). Genetic control models with diffusion and delays. Math. Biosci., 90, 519–533.
Mahaffy, J., & Pao, C. (1984). Models of genetic control by repression with time delays and spatial effects. J. Math. Biol., 20, 39–57.
Maki, C., Huibregtse, J., & Howley, P. (1996). In vivo ubiquitination and proteasome-mediated degradation of p53. Cancer Res., 56(11), 2649–2654.
Marfori, M., Mynott, A., Ellis, J. J., Mehdi, A. M., Saunders, N. F. W., Curmi, P. M., Forwood, J. K., Boden, M., & Kobe, B. (2011). Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim. Biophys. Acta, Mol. Cell Res., 1813(9), 1562–1577.
Masamizu, Y., Ohtsuka, T., Takashima, Y., Nagahara, H., Takenaka, Y., Yoshikawa, K., Okamura, H., & Kageyama, R. (2006). Real-time imaging of the somite segmentation clock: revelation f unstable oscillators in the individual presomitic mesoderm cells. Proc. Natl. Acad. Sci. USA, 103, 1313–1318.
Matsuda, T., Miyawaki, A., & Nagai, T. (2008). Direct measurement of protein dynamics inside cells using a rationally designed photoconvertible protein. Nat. Methods, 5, 339–345.
Mayo, L., & Donner, D. (2001). A phosphatidylinositol 3-kinase/akt pathway promotes translocation of mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA, 98(20), 11598–11603.
Mendez, V., Fedotov, S., & Horsthemke, W. (2010). Reaction-transport systems. Berlin: Springer.
Meyers, J., Craig, J., & Odde, D. (2006). Potential for control of signaling pathways via cell size and shape. Curr. Biol., 16, 1685–1693.
Michalet, X., Pinaudand, F., Bentolila, L., Tsay, J., Doose, S., Li, J., Sundaresan, G., Wu, A., Gambhir, S., & Weiss, S. (2005). Quantum dots for live cells, in vivo imaging, and diagnostics. Science, 307, 538–544.
Mihalas, G., Neamtu, M., Opris, D., & Horhat, R. (2006). A dynamic P53–MDM2 model with time delay. Chaos Solitons Fractals, 30, 936–945.
Momiji, H., & Monk, N. (2008). Dissecting the dynamics of the Hes1 genetic oscillator. J. Theor. Biol., 254, 784–798.
Monk, N. (2003). Oscillatory expression of Hes1, p53, and NF-κB driven by transcriptional time delays. Curr. Biol., 13, 1409–1413.
Muller, M., Klumpp, S., & Lipowsky, R. (2008). Tug-of-war as a cooperative mechanism for bidirectional cargo transport of molecular motors. Proc. Natl. Acad. Sci. USA, 105, 4609–4614.
Nelson, D., Ihekwaba, A., Elliott, M., Johnson, J., Gibney, C., Foreman, B., Nelson, G., See, V., Horton, C., Spiller, D., Edwards, S., McDowell, H., Unitt, J., Sullivan, E., Grimley, R., Benson, N., Broomhead, D., Kell, D., & White, M. (2004). Oscillations in NF-κB signaling control the dynamics of gene expression. Science, 306, 704–708.
Neves, S., Tsokas, P., Sarkar, A., Grace, E., Rangamani, P., Taubenfeld, S., Alberini, C., Schaff, J., Blitzer, R., Moraru, I., & Iyengar, R. (2008). Cell shape and negative links in regulatory motifs together control spatial information flow in signaling networks. Cell, 133, 666–680.
Norvell, A., Debec, A., Finch, D., Gibson, L., & Thoma, B. (2005). Squid is required for efficient posterior localization of oskar mRNA during drosophila oogenesis. Dev. Genes Evol., 215, 340–349.
O’Brate, A., & Giannakakou, P. (2003). The importance of p53 location: nuclear or cytoplasmic zip code? Drug Resist. Updat., 6(6), 313–322.
Orlowski, R., & Kuhn, D. (2008). Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin. Cancer Res., 14, 1649–1657.
Ouattara, D., Abou-Jaoudé, W., & Kaufman, M. (2010). From structure to dynamics: frequency tuning in the p53–Mdm2 network. II. Differential and stochastic approaches. J. Theor. Biol., 264, 1177–1189.
Perkins, N. (2007). Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol., 8, 49–62.
Pincus, Z., & Theriot, J. (2007). Comparison of quantitative methods for cell-shape analysis. J. Microsc., 227(2), 140–156.
Pommier, Y., Sordet, O., Antony, S., Hayward, R., & Kohn, K. (2004). Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene, 23, 2934–2949.
Prinz, H. (2010). Hill coefficients, dose-response curves, and allosteric mechanisms. J. Chem. Biol., 3, 37–44.
Proctor, C., & Gray, D. (2008). Explaining oscillations and variability in the p53–Mdm2 system. BMC Syst. Biol., 2(75), 1–20.
Puszyński, K., Bertolusso, R., & Lipniacki, T. (2009). Crosstalk between p53 and NF-κB systems: pro- and anti-apoptotic functions of NF-κB. IET Syst. Biol., 3, 356–367.
Puszyński, K., Hat, B., & Lipniacki, T. (2008). Oscillations and bistability in the stochastic model of p53 regulation. J. Theor. Biol., 254, 452–465.
Rangamani, P., & Iyengar, R. (2007). Modelling spatio-temporal interactions within the cell. J. Biosci., 32, 157–167.
Robati, M., Holtz, D., & Dunton, C. J. (2008). A review of topotecan in combination chemotherapy for advanced cervical cancer. Ther. Clin. Risk. Manag., 4, 213–218.
Rodriguez, M. S., Dargemont, C., & Stutz, F. (2004). Nuclear export of RNA. Biol. Cell, 96, 639–655.
Roth, D., Moseley, G., Glover, D., Pouton, C., & Jans, D. (2007). A microtubule-facilitated nuclear import pathway for cancer regulatory proteins. Traffic, 8(6), 673–686.
Ryan, K., Phillips, A., & Vousden, K. (2001). Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol., 13, 332–337.
Sang, L., Coller, H., & Roberts, J. (2008). Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science, 321, 1095–1100.
Seksek, O., Biwersi, J., & Verkman, A. (1997). Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J. Cell Biol., 138, 131–142.
Shahrezaei, V., & Swain, P. (2008). The stochastic nature of biochemical networks. Curr. Opin. Biotechnol., 19, 369–374.
Shankaran, H., Ippolito, D., Chrisler, W., Resat, H., Bollinger, N., Opresko, L., & Wiley, H. (2009). Rapid and sustained nuclear-cytoplasmic ERK oscillations induced by epidermal growth factor. Mol. Syst. Biol., 5, 322.
Shymko, R., & Glass, L. (1974). Spatial switching in chemical reactions with heterogeneous catalysis. J. Chem. Phys., 60, 835–841.
Smith, D., & Simmons, R. (2001). Model of motor-assisted transport of intracelullar particules. Biophys. J., 80, 45–68.
Sturrock, M., Terry, A., Xirodimas, D., Thompson, A., & Chaplain, M. (2011). Spatio-temporal modelling of the Hes1 and p53–Mdm2 intracellular signalling pathways. J. Theor. Biol., 273, 15–31.
Terry, A., & Chaplain, M. (2011). Spatio-temporal modelling of the NF-κB intracellular signalling pathway: the roles of diffusion, active transport, and cell geometry. J. Theor. Biol., 290, 7–26.
Terry, A., Sturrock, M., Dale, J., Maroto, M., & Chaplain, M. (2011). A spatio-temporal model of Notch signalling in the zebrafish segmentation clock: conditions for synchronised oscillatory dynamics. PLoS ONE, 6, e16980.
Thut, C., Goodrich, J., & Tjian, R. (1997). Repression of p53-mediated transcription by MDM2: a dual mechanism. Genes Dev., 11, 1974–1986.
van Zon, J., Morelli, M., Tănase-Nicola, S., & ten Wolde, P. (2006). Diffusion of transcription factors can drastically enhance the noise in gene expression. Biophys. J., 91, 4350–4367.
Wachsmuth, M., Waldeck, W., & Langowski, J. (2000). Anomalous diffusion of fluorescent probes inside living cell nuclei investigated by spatially-resolved fluorescence correlation spectroscopy. J. Mol. Biol., 298, 677–689.
Wang, B., Xiao, Z., Ko, H. L., & Ren, E. C. (2010). The p53 response element and transcriptional repression. Cell Cycle, 9(5), 870–879.
Weis, K. (2003). Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell, 112, 441–451.
Weiss, M., Hashimoto, H., & Nilsson, T. (2004). Anomalous protein diffusion is a measure for cytoplasmic crowding in living cells. Biophys. J., 87, 3518–3524.
Xirodimas, D., Stephen, C., & Lane, D. (2001). Cocompartmentalization of p53 and Mdm2 is a major determinant for Mdm2-mediated degradation of p53. Exp. Cell Res., 270, 66–77.
Zeiser, S., Muller, J., & Liebscher, V. (2007). Modeling the Hes1 oscillator. J. Comput. Biol., 14, 984–1000.
Zhang, P., Yang, Y., Zweidler-McKay, P. A., & Hughes, D. (2008). Critical role of notch signaling in osteosarcoma invasion and metastasis. Clin. Cancer Res., 14, 2962–2969.
Zhang, T., Brazhnik, P., & Tyson, J. (2007). Exploring mechanisms of the DNA-damage response: p53 pulses and their possible relevance to apoptosis. Cell Cycle, 6, 85–94.
Acknowledgements
The authors gratefully acknowledge the support of the ERC Advanced Investigator Grant 227619, “M5CGS—From Mutations to Metastases: Multiscale Mathematical Modelling of Cancer Growth and Spread”.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below are the links to the electronic supplementary material.
(MP4 1.0 MB)
(MP4 1.0 MB)
Appendix
Appendix
1.1 A.1 Non-dimensionalisation of Hes1 Model
We summarise our non-dimensionalisation of the extended Hes1 model (described in Sect. 2.2). The original Hes1 model (described in Sect. 2.1) is non-dimensionalised in a similar way; for details, see Sturrock et al. (2011).
To non-dimensionalise the extended Hes1 model given by (1)–(4) and (15), subject to the conditions in (8)–(14), we first define re-scaled variables by dividing each variable by a reference value. Re-scaled variables are given overlines to distinguish them from variables that are not re-scaled. Thus we can write:

where the right-hand side of each equation is a dimensional variable divided by its reference value. From (47), we can write variables in terms of re-scaled variables and then substitute these expressions into (1)–(4) and (15), and into the conditions in (8)–(14). This gives a model defined in terms of re-scaled variables which has the same form as the dimensional model, but now the parameters are all non-dimensional. Denoting the non-dimensional parameters with an asterisk, they are related to dimensional parameters as follows:

We solve the non-dimensional model using the method described in Sect. 2.1. We simulate the model in COMSOL 3.5a, finding non-dimensional parameter values that yield oscillatory dynamics. We chose the same values as in Eq. (25) in Sturrock et al. (2011) except for those parameters which were new because of our extension to the model. These latter values were chosen as follows: \(D^{*}_{m} = D^{*}_{i_{j}}/5\), \(D^{*}_{p} = D^{*}_{i_{j}}/15\), d ∗=0.01, a ∗=0.03, l ∗=0.63.
Finally, we calculated the dimensional parameter values. To do this, we needed to estimate the reference values. Since Her1 in zebrafish and Hes1 in mice are both pathways connected with somitogenesis, we used the reference concentrations for Her1 protein and her1 mRNA in Terry et al. (2011) as our reference concentrations for Hes1 protein and hes1 mRNA. Thus, we chose [m 0]=1.5×10−9 M and [p 0]=10−9 M. We assumed a cell to be of width 30 μm. But from Figs. 2 and 4, the cell width is equal to three non-dimensional spatial units or 3L-dimensional units (using (47)). Hence we set 3L=30 μm, so that L=10 μm. The experimentally observed period of oscillations of Hes1 is approximately 2 hours (Hirata et al. 2002). Our simulations of the non-dimensionalised model gave oscillations with a period of approximately 300 non-dimensional time units or 300 τ-dimensional units (using (47)). Hence we set 300τ=2 h=7200 s, so that τ=24 s. Using our references values and non-dimensional parameter values, we found dimensional parameter values from (48).
Note that we chose our reference time τ=24 s based on simulations of the extended Hes1 model since this was our most realistic Hes1 model. For the original Hes1 model and for all special cases of the Hes1 model (for example, setting active transport rates to zero), we retained the reference time τ=24 s.
1.2 A.2 Non-dimensionalisation of p53–Mdm2 Model
We non-dimensionalised the p53–Mdm2 model defined in Sect. 3.1, and the extended p53–Mdm2 model defined in Sect. 3.2, using the technique described above for non-dimensionalising the extended Hes1 model. We give brief details for our non-dimensionalisation of the extended p53–Mdm2 model.
To non-dimensionalise the extended p53–Mdm2 model given by (19)–(26) and (44)–(45), subject to conditions (27) and (32)–(43), we define re-scaled variables (denoted by overlines) by dividing each variable by a reference value:

Substituting the scaling in (49) into the extended p53–Mdm2 model gives a non-dimensionalised model with non-dimensional parameters (which we denote with asterisks) that are related to dimensional parameters as follows:

We solve the non-dimensional model using COMSOL 3.5a, finding non-dimensional parameter values that yield oscillatory dynamics. We chose the same values as in Eq. (60) in Sturrock et al. (2011) except for those parameters which were new because of our extension to the model. These latter values were chosen as follows: θ ∗=1, ζ ∗=0.35, \(D^{*}_{m} = D^{*}_{i_{j}}/5\), \(D^{*}_{p} =D^{*}_{i_{j}}/15\), d ∗=0.01, a ∗=0.03, l ∗=0.63.
Finally, we calculated the dimensional parameter values. To do this, we had to estimate the reference values. As in Sturrock et al. (2011), we chose the following reference concentrations: [p530]=0.5 μM, [Mdm2m 0]=0.05 μM, [Mdm20]=2 μM. In addition, we chose [p53m 0]=0.025 μM (in keeping with relative concentrations of mRNA and protein revealed by simulation of the non-dimensionalised model). As with the Hes1 model, we assumed a cell to be of width 30 μm, which again leads to the reference length L=10 μm. Our simulations of the non-dimensionalised model gave oscillations with a period of approximately 360 non-dimensional time units or 360 τ-dimensional units (using (50)), and the experimentally observed period is approximately 3 hours (Monk 2003). Hence we set 360τ=3 h=10800 s, so that τ=30 s. The reference time τ=30 s was based on simulations of the extended p53–Mdm2 model since this was our most realistic p53–Mdm2 model. For all variants of this model (for example, setting active transport rates to zero), we retained the reference time τ=30 s for ease of comparison of the numerical results. Using our references values and non-dimensional parameter values, we found dimensional parameter values from (50).
1.3 A.3 Influence of Cell Shape
As discussed in Sect. 2.2.5, we carried out simulations for the extended Hes1 model on a variety of cell geometries. Results from these simulations are presented in Figs. 24 and 25. It is clear from these figures that sustained oscillatory dynamics are strongly robust to changes in cell shape. Such robustness is reassuring since the shape of eukaryotic cells is highly variable (Baserga 2007; Pincus and Theriot 2007).
Plots showing the effect on the extended Hes1 model of varying the nuclear shape. In each row, the left plot shows the shape on which we solve, and the middle and right plots show the corresponding numerical results. Spatial units here are non-dimensional, with one non-dimensional spatial unit corresponding to 10 μm. Total concentrations for Hes1 protein are displayed in blue and for hes1 mRNA in red. Parameter values as per column 2 of Table 2
Plots showing the effect on the extended Hes1 model of varying the nucleus position (first row), the MTOC position (second row), and the cell shape (third row). In each row, the left plot shows the shape on which we solve, and the middle and right plots show the corresponding numerical results. Spatial units here are non-dimensional, with one non-dimensional spatial unit corresponding to 10 μm. Total concentrations for Hes1 protein are displayed in blue and for hes1 mRNA in red. Parameter values as per column 2 of Table 2
Only one of the geometries in Figs. 24 or 25 shows significant damping after the initial peaks in Hes1 protein and hes1 mRNA total concentrations. This occurs in the second row in Fig. 25, where the MTOC surrounding the nucleus is significantly increased in size. The increased size of the MTOC reduces the size of the region in which active transport may occur. Hence the results in the second row in Fig. 25 are similar to those presented in Sect. 2.2.4 in which the active transport rate is set to zero.
Rights and permissions
About this article
Cite this article
Sturrock, M., Terry, A.J., Xirodimas, D.P. et al. Influence of the Nuclear Membrane, Active Transport, and Cell Shape on the Hes1 and p53–Mdm2 Pathways: Insights from Spatio-temporal Modelling. Bull Math Biol 74, 1531–1579 (2012). https://doi.org/10.1007/s11538-012-9725-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-012-9725-1