Abstract
Background
The decrease in carcinoembryonic antigen (CEA) level is faster and greater during cetuximab treatment than bevacizumab treatment and correlates with prolonged survival in patients with metastatic colorectal cancer (mCRC) who receive cetuximab.
Objective
We investigated if the degree of change in the CEA value can serve as a diagnostic tool for predicting survival, as well as tumor regression in mCRC patients treated with cetuximab combined regimen as first-line treatment.
Patients and Methods
Associations among the CEA decrease, depth of response (DpR), and clinical outcomes were evaluated in 113 patients with mCRC from two phase II trials of first-line therapy: the JACCRO CC-05 trial of cetuximab plus FOLFOX and the CC-06 trial of cetuximab plus SOX. Analysis was performed using Spearman’s rank correlation coefficient. A 75% decrease in the CEA was used as the cut-off value to define the CEA response and discriminate CEA responders on the basis of the results of a previous study.
Results
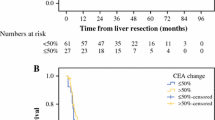
Ninety-two patients were eligible for analyses of both CEA and DpR. The median CEA decrease was 67.4%, and the median time to CEA nadir was 2.8 months, which was similar to the median time to DpR of 3.0 months. The DpR was associated with PFS and OS (rs = 0.56, P < 0.0001; rs = 0.39, P = 0.0090, respectively); moreover, the CEA decrease correlated with PFS (rs = 0.56, P < 0.0001), as well as OS (rs = 0.35, P = 0.019). CEA responders had significantly longer PFS (11.8 vs. 5.5 months, hazard ratio [HR] 0.46, P = 0.0009) and slightly, but not significantly longer OS (36.2 vs. 23.5 months; HR 0.57; P = 0.072) than CEA non-responders. The CEA decrease was statistically significantly associated with the DpR (rs = 0.44, P < 0.0001).
Conclusions
Our study demonstrates that both DpR and CEA response correlate with clinical outcomes of first-line treatment with cetuximab. The CEA decrease may serve as a surrogate for DpR in patients who receive first-line cetuximab treatment (UMIN000004197, UMIN000007022).





Similar content being viewed by others
References
De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, et al. Association of KRAS p.G13D Mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–20.
Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–34.
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75.
Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–94.
Carriquiry LA, Pineyro A. Should carcinoembryonic antigen be used in the management of patients with colorectal cancer? Dis Colon Rectum. 1999;42:921–9.
Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122:467–81.
Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, et al. Tumour markers in colorectal cancer: European group on tumour markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43:1348–60.
Fortner JG, Silva JS, Golbey RB, Cox EB, Maclean BJ. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. I. Treatment by hepatic resection. Ann Surg. 1984;199:306–16.
Dhar P, Moore T, Zamcheck N, Kupchik HZ. Carcinoembryonic antigen (CEA) in colonic cancer. Use in preoperative and postoperative diagnosis and prognosis. JAMA. 1972;221:31–5.
Duffy MJ, van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R, et al. Clinical utility of biochemical markers in colorectal cancer: European group on tumour markers (EGTM) guidelines. Eur J Cancer. 2003;39:718–27.
Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–27.
Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1453–86.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422.
Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, Cooper HS, et al. NCCN clinical practice guidelines in oncology: colon cancer. J Natl Compr Cancer Netw. 2009;7:778–831.
Preketes AP, King J, Caplehorn JR, Clingan PR, Ross WB, Morris DL. CEA reduction after cryotherapy for liver metastases from colon cancer predicts survival. Aust N Z J Surg. 1994;64:612–4.
Ward U, Primrose JN, Finan PJ, Perren TJ, Selby P, Purves DA, et al. The use of tumour markers CEA, CA-195 and CA-242 in evaluating the response to chemotherapy in patients with advanced colorectal cancer. Br J Cancer. 1993;67:1132–5.
Iwanicki-Caron I, Di Fiore F, Roque I, Astruc E, Stetiu M, Duclos A, et al. Usefulness of the serum carcinoembryonic antigen kinetic for chemotherapy monitoring in patients with unresectable metastasis of colorectal cancer. J Clin Oncol. 2008;26:3681–6.
Michl M, Koch J, Laubender RP, Modest DP, Giessen C, Schulz C, et al. Tumor markers CEA and CA 19-9 correlate with radiological imaging in metastatic colorectal cancer patients receiving first-line chemotherapy. Tumour Biol. 2014;35:10121–7.
Huang SC, Lin JK, Lin TC, Chen WS, Yang SH, Wang HS, et al. Concordance of Carcinoembryonic antigen ratio and response evaluation criteria in solid tumors as prognostic surrogate indicators of metastatic colorectal cancer patients treated with chemotherapy. Ann Surg Oncol. 2015;22:2262–8.
Michl M, Stintzing S, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, et al. CEA response is associated with tumor response and survival in patients with KRAS exon 2 wild-type and extended RAS wild-type metastatic colorectal cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial). Ann Oncol. 2016;27:1565–72.
Heinemann V, Stintzing S, Modest DP, Giessen-Jung C, Michl M, Mansmann UR. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer. 2015;51:1927–36.
Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26:1188–94.
Mansmann UR, Laubender RP. Methodologic diligence is needed to define and validate tumor-size response metrics to predict overall survival in first-line metastatic colorectal cancer. J Clin Oncol. 2013;31:4373–4.
Mayer RJ, Garnick MB, Steele GD Jr, Zamcheck N. Carcinoembryonic antigen (CEA) as a monitor of chemotherapy in disseminated colorectal cancer. Cancer. 1978;42:1428–33.
Fakih MG, Padmanabhan A. CEA monitoring in colorectal cancer. What you should know. Oncology (Williston Park). 2006;20:579–87.
Missiaglia E, Jacobs B, D’Ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001.
Heinemann V, Modest DP, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, et al. Gender and tumor location as predictors for efficacy: influence on endpoints in first-line treatment with FOLFIRI in combination with cetuximab or bevacizumab in the AIO KRK 0306 (FIRE3) trial. J Clin Oncol. 2014;32(5 suppl; abstr 3600).
von Einem JC, Heinemann V, von Weikersthal LF, Vehling-Kaiser U, Stauch M, Hass HG, et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: an analysis of the AIO KRK-0104 trial. J Cancer Res Clin Oncol. 2014;140:1607–14.
Sunakawa Y, Ichikawa W, Tsuji A, Denda T, Segawa Y, Negoro Y, et al. Prognostic impact of primary tumor location on clinical outcomes of metastatic colorectal cancer treated with Cetuximab plus Oxaliplatin-based chemotherapy: a subgroup analysis of the JACCRO CC-05/06 trials. Clin Colorectal Cancer. 2017;16:e171–e180.
Acknowledgements
We thank the patients, their families, and the investigators who participated in the JACCRO CC-05 and CC-06 trials. We also thank Dr. Toshifusa Nakajima for trial support, Sachika Koyama for editorial assistance, and Peter Star (Medical Network K.K.) for English editorial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee (or both) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This study was supported by the Japan Clinical Cancer Research Organization (JACCRO).
Conflict of Interest
Author Y.S. has received honoraria from Taiho Pharmaceutical, Chugai Pharma, Yakult Honsha, Takeda, and Merck Serono. Author W.I. has received consulting fees from Merck Serono, Daiichi Sankyo, Zeria Pharmaceutical, and Ono Pharmaceutical; research funding from Merck Serono, Taiho Pharmaceutical, Takeda, and Eisai; and honoraria from Taiho Pharmaceutical, Merck Serono, Chugai Pharma, Daiichi Sankyo, Takeda, Nippon Kayaku and Sawai Pharmaceutical Co. Author A.T. has received honoraria from Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma, and Merck Serono. Author M.N. has received honoraria from Merck Serono, Takeda, Chugai Pharma, Taiho Pharmaceutical, Nippon Kayaku, Novartis, Yakult Honsha, Lilly Japan, Bristol-Myers Squibb, Bayer, Ajinomoto, Shionogi, Pfizer, and Ono Pharmaceutical. Author M.K. has received honoraria from Merck Serono, Takeda, Chugai Pharma, and Yakult Honsha. Author M.T. has received consulting fees from Taiho Pharmaceutical, Shionogi, AbbVie GK, Astra Zeneca Co., and Hisamitsu Pharma Co.; and honoraria from Mitsubishi Tanabe Pharma. The other authors have declared no conflicts of interest.
Electronic supplementary material
ESM 1
(PDF 80 kb)
Rights and permissions
About this article
Cite this article
Sunakawa, Y., Tsuji, A., Denda, T. et al. CEA Response and Depth of Response (DpR) to Predict Clinical Outcomes of First-Line Cetuximab Treatment for Metastatic Colorectal Cancer. Targ Oncol 12, 787–794 (2017). https://doi.org/10.1007/s11523-017-0527-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-017-0527-0