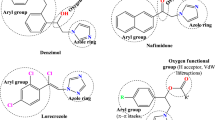
Abstract
Background
Among the reported potential agents to treat the epilepsy, sulphonamides are important and their significance cannot be ignored. A series of substituted 4-amino-benzene sulfonamides were designed, keeping in view the structural requirement of pharmacophore.
Methods
Lipinski rule of five has been calculated; failure to Lipinski rule was not observed. Docking was performed through AutoDock Vina. Molecules have been screened out through docking. Compounds were synthesized and characterized through IR, 1HNMR, 13C NMR, Mass and elemental analysis. The anticonvulsant activity of the synthesized compounds was assessed using the Maximal Electroshock Seizure (MES) model. In-silico biological activity spectrum, toxicological studies, predicted oral rats LD50 were performed.
Results
Docking studies showed good interaction with lyase (Oxo-acid) - human carbonic anhydrase-I (1AZM). The in-silico studies proved them to be with good drug-likeness properties, especially 4-(3-Acetyl-phenylamino)-methyl)-benzenesulfonamide (2g). These results revealed that the synthesized compounds (1a-1c, 2a-2q) exhibited promising anticonvulsant effect against MES model for inhibition of Lyase- Human Carbonic Anhydrase-I.
Conclusion
After investigating all the results, the compound 4-(3-Acetyl-phenylamino)-methyl)-benzenesulfonamide (2g) is found to be best in the series. A comparatively good activity of compound 2g suggests us that sulphonamide can be leads to further optimization for building potent and chemically diversified anti-convulsant agents.
Similar content being viewed by others
References
Barry Wood W (1942). Studies on the antibacterial action of the sulfonamide drugs. J Exp Med, 75(4): 369–381
Bialk H M, Simpson A J, Pedersen J A (2005). Cross-coupling of sulfonamide antimicrobial agents with model humic constituents. Environ Sci Technol, 39(12): 4463–4473
Capasso C, Supuran C T (2015). An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem, 30(2): 325–332
De Simone G, Scozzafava A, Supuran C T (2009). Which carbonic anhydrases are targeted by the antiepileptic sulfonamides and sulfamates? Chem Biol Drug Des, 74(3): 317–321
Filimonov D A, Poroikov V V, Karaicheva E I, Kazarian R K, Budunova A P, Mikhailovskii E M, Rudnitskikh A V, Goncharenko L V, Burov Yu V (1995). Computer-Aided Prediction of Biological Activity Spectra of Chemical Substances on the Basis of Their Structural Formulae: Computerized System PASS. Experimental and Clinical Pharmacology(Rus), 58(2): 56–62
Hen N, Bialer M, Wlodarczyk B, Finnell R H, Yagen B (2010). Syntheses and evaluation of anticonvulsant profile and teratogenicity of novel amide derivatives of branched aliphatic carboxylic acids with 4-aminobenzensulfonamide. J Med Chem, 53(10): 4177–4186
Leeson P (2012). Drug discovery: Chemical beauty contest. Nature, 481 (7382): 455–456
Malawska B, Scatturin A (2003). Application of pharmacophore models for the design and synthesis of new anticonvulsant drugs. Mini Rev Med Chem, 3(4): 341–348
Masereel B, Rolin S, Abbate F, Scozzafava A, Supuran C T (2002). Carbonic anhydrase inhibitors: anticonvulsant sulfonamides incorporating valproyl and other lipophilic moieties. J Med Chem, 45(2): 312–320
Perrin H L, Bliss E A (1937). Para-aminobenzenesulfonamide and its derivatives experimental and clinical observations on their use in the treatment of betahemolytic streptococcic infection: a preliminary report. J. Am. Med. Soc., 108(1): 32–37
Price T, Sammons G, Zachry D (1952). Antitubercular Studies. V. 4- Aminobenzamides and 4-Aminobenzenesulfonamides. J Am Chem Soc, 74(23): 5961–5963
Reynolds C H, Merz K M, Ringe D (2010). Drug Design: Structure- And Ligand-Based Approaches, Cambridge University Press, Cambridge UK
Reynolds E H (2002). Epilepsy in the world: Launch of the second phase of the ILAE/IBE/WHO global campaign against epilepsy. Epilepsia, 43(supp. 6): 1–3
Satischandra P, Gururaj G, Mohammed Q D, Senanayake N, Silpakit O, Dekker P A (2005). Epilepsy: A manual for physicians. World Health Organization, New Delhi. 1–15
Supuran C T (2008). Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov, 7(2): 168–181
Thiry A, Dogné J M, Supuran C T, Masereel B (2007). Carbonic anhydrase inhibitors as anticonvulsant agents. Curr Top Med Chem, 7(9): 855–864
Thiry A, Dogné J M, Supuran C T, Masereel B (2008). Anticonvulsant sulfonamides/sulfamates/sulfamides with carbonic anhydrase inhibitory activity: drug design and mechanism of action. Curr Pharm Des, 14(7): 661–671
Tripathi L, Kumar P, Singh R, Stables J P (2012). Design, synthesis and anticonvulsant evaluation of novel N-(4-substituted phenyl)-2-(4- (substituted) benzylidene)-hydrazinecarbothio amides. Eur J Med Chem, 47(1): 153–166
Trott O, Olson A J (2010). AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem, 31(2): 455–461
Vannada J, Bennett E M, Wilson D J, Boshoff H I, Barry C E 3rd, Aldrich C C (2006). Design, synthesis, and biological evaluation of beta-ketosulfonamide adenylation inhibitors as potential antitubercular agents. Org Lett, 8(21): 4707–4710
Venkatapathy R, Moudgal C J, Bruce R M (2004). Assessment of the oral rat chronic lowest observed adverse effect level model in TOPKAT, a QSAR software package for toxicity prediction. J Chem Inf Comput Sci, 44(5): 1623–1629
Zimmerman S, Innocenti A, Casini A, Ferry J G, Scozzafava A, Supuran C T (2004). Carbonic anhydrase inhibitors. Inhibition of the prokariotic beta and gamma-class enzymes from Archaea with sulfonamides. Bioorg Med Chem Lett, 14(24): 6001–6006
Acknowledgment
One of the authors (Ajeet) is thankful to Ms. Pratibha Priti Maurya, Owner, CBBE, India for in-silico studies, and thankful to Dr. Om Prakash, Post Doctorate Fellow, University of Lucknow, India for interpretation and support of In-silico studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ajeet, Kumar, A. & Mishra, A.K. Design, molecular docking, synthesis, characterization, biological activity evaluation (against MES model), in-silico biological activity spectrum (PASS analysis), toxicological and predicted oral rat LD50 studies of novel sulphonamide derivatives. Front. Biol. 13, 425–451 (2018). https://doi.org/10.1007/s11515-018-1512-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-018-1512-4