Abstract
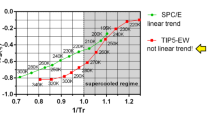
Classical molecular dynamics simulations were performed to study the high-temperature (above 300 K) dynamic behavior of bulk water, specifically the behavior of the diffusion coefficient, hydrogen bond, and nearest-neighbor lifetimes. Two water potentials were compared: the recently proposed “globally optimal” point charge (OPC) model and the well-known TIP4P-Ew model. By considering the Arrhenius plots of the computed inverse diffusion coefficient and rotational relaxation constants, a crossover from Vogel–Fulcher–Tammann behavior to a linear trend with increasing temperature was detected at T* ≈ 309 and T* ≈ 285 K for the OPC and TIP4P-Ew models, respectively. Experimentally, the crossover point was previously observed at T* ± 315–5 K. We also verified that for the coefficient of thermal expansion α P (T, P), the isobaric α P (T) curves cross at about the same T* as in the experiment. The lifetimes of water hydrogen bonds and of the nearest neighbors were evaluated and were found to cross near T*, where the lifetimes are about 1 ps. For T < T*, hydrogen bonds persist longer than nearest neighbors, suggesting that the hydrogen bonding network dominates the water structure at T < T*, whereas for T > T*, water behaves more like a simple liquid. The fact that T* falls within the biologically relevant temperature range is a strong motivation for further analysis of the phenomenon and its possible consequences for biomolecular systems.
Similar content being viewed by others
References
P. Ball, Water: Water an enduring mystery, Nature 452 (7185), 291 (2008)
P. Gallo, K. Amann-Winkel, C. A. Angell, M. A. Anisimov, F. Caupin, C. Chakravarty, E. Lascaris, T. Loerting, A. Z. Panagiotopoulos, J. Russo, J. A. Sellberg, H. E. Stanley, H. Tanaka, C. Vega, L. Xu, and L. G. M. Pettersson, Water: A tale of two liquids, Chem. Rev. 116(13), 7463 (2016)
A. Nilsson and L. G. M. Pettersson, The structural origin of anomalous properties of liquid water, Nat. Commun. 6, 8998 (2015)
H. E. Stanley, Advances in Chemical Physics: Liquid Polymorphism, Vol. 152, John Wiley & Sons, 2013
J. H. Simpson and H. Y. Carr, Diffusion and nuclear spin relaxation in water, Phys. Rev. 111(5), 1201 (1958)
F. Mallamace, C. Corsaro, and H. E. Stanley, A singular thermodynamically consistent temperature at the origin of the anomalous behavior of liquid water, Sci. Rep. 2, 993 (2012)
F. Mallamace, C. Corsaro, D. Mallamace, C. Vasi, and H. E. Stanley, The thermodynamical response functions and the origin of the anomalous behavior of liquid water, Faraday Discuss. 167, 95 (2013)
F. Mallamace, C. Corsaro, D. Mallamace, S. Vasi, C. Vasi, and H. E. Stanley, Thermodynamic properties of bulk and confined water, J. Chem. Phys. 141(18), 18C504 (2014)
H. R. Pruppacher, Self-Diffusion coefficient of supercooled water, J. Chem. Phys. 56(1), 101 (1972)
NIST Chemistry WebBook, 2008. http://webbook.nist.gov/chemistry/uid/
F. Mallamace, C. Corsaro, D. Mallamace, S. Vasi, C. Vasi, H. E. Stanley, and S. H. Chen, Some thermodynamical aspects of protein hydration water, J. Chem. Phys. 142(21), 215103 (2015)
R. Speedy and C. Angell, Isothermal compressibility of supercooled water and evidence for a thermodynamic singularity at ??45 ?C, J. Chem. Phys. 65(3), 851 (1976)
P. W. Bridgman, Water, in the liquid and five solid forms, under pressure, in: Proceedings of the American Academy of Arts and Sciences, pp 441–558, JSTOR, 1912
G. S. Kell, Density, thermal expansivity, and compressibility of liquid water from 0°C to 150°C: Correlations and tables for atmospheric pressure and saturation reviewed and expressed on 1968 temperature scale, J. Chem. Eng. Data 20(1), 97 (1975)
G. Kell and E. Whalley, Reanalysis of the density of liquid water in the range 0–150 °C and 0–1 kbar, J. Chem. Phys. 62(9), 3496 (1975)
C. Sorensen, Densities and partial molar volumes of supercooled aqueous solutions, J. Chem. Phys. 79(3), 1455 (1983)
D. Hare and C. Sorensen, Densities of supercooled H2O and D2O in 25 glass capillaries, J. Chem. Phys. 84(9), 5085 (1986)
D. Hare and C. Sorensen, The density of supercooled water (II): Bulk samples cooled to the homogeneous nucleation limit, J. Chem. Phys. 87(8), 4840 (1987)
O. Mishima, Volume of supercooled water under pressure and the liquid-liquid critical point, J. Chem. Phys. 133(14), 144503 (2010)
W. D. Wilson, Speed of sound in distilled water as a function of temperature and pressure, J. Acoust. Soc. Am. 31(8), 1067 (1959)
R. C. Dougherty and L. N. Howard, Equilibrium structural model of liquid water: Evidence from heat capacity, spectra, density, and other properties, J. Chem. Phys. 109(17), 7379 (1998)
H. Vogel, The law of the relation between the viscosity of liquids and the temperature, Phys. Z. 22, 645 (1921)
G. S. Fulcher, Analysis of recent measurements of the viscosity of glasses, J. Am. Ceram. Soc. 8(6), 339 (1925)
G. Tammann and W. Hesse, The dependence of viscosity upon the temperature of supercooled liquids, Z. Anorg. Allg. Chem. 156, 245 (1926)
W. S. Price, H. Ide, and Y. Arata, Self-Diffusion of supercooled water to 238 K using PGSE NMR diffusion measurements, J. Phys. Chem. A 103(4), 448 (1999)
D. Laage and J. T. Hynes, A molecular jump mechanism of water reorientation, Science 311 (5762), 832 (2006)
D. Laage and J. T. Hynes, On the molecular mechanism of water reorientation, J. Phys. Chem. B 112(45), 14230 (2008)
G. Stirnemann and D. Laage, Direct evidence of angular jumps during water reorientation through twodimensional infrared anisotropy, J. Phys. Chem. Lett. 1(10), 1511 (2010)
D. Laage, G. Stirnemann, F. Sterpone, and J. T. Hynes, Water jump reorientation: from theoretical prediction to experimental observation, Acc. Chem. Res. 45(1), 53 (2012)
D. Laage, G. Stirnemann, F. Sterpone, R. Rey, and J. T. Hynes, Reorientation and allied dynamics in water and aqueous solutions, Annu. Rev. Phys. Chem. 62(1), 395 (2011)
L. B. Skinner, C. J. Benmore, J. C. Neuefeind, and J. B. Parise, The structure of water around the compressibility minimum, J. Chem. Phys. 141(21), 214507 (2014)
D. Schlesinger, K. T. Wikfeldt, L. B. Skinner, C. J. Benmore, A. Nilsson, and L. G. M. Pettersson, The temperature dependence of intermediate range oxygen-oxygen correlations in liquid water, J. Chem. Phys. 145(8), 084503 (2016)
F. Mallamace, C. Corsaro, D. Mallamace, S. Vasi, C. Vasi, and G. Dugo, The role of water in protein’s behavior: The two dynamical crossovers studied by NMR and FTIR techniques, Comput. Struct. Biotechnol. J. 13, 33 (2015)
P. Demontis, J. Gulín-González, M. Masia, M. Sant, and G. B. Suffritti, The interplay between dynamic heterogeneities and structure of bulk liquid water: A molecular dynamics simulation study, J. Chem. Phys. 142(24), 244507 (2015)
H. W. Horn, W. C. Swope, J. W. Pitera, J. D. Madura, T. J. Dick, G. L. Hura, and T. Head-Gordon, Development of an improved four-site water model for biomolecular simulations: TIP4P-Ew, J. Chem. Phys. 120 (20), 9665 (2004)
P. Demontis, J. Gulín-González, M. Masia, and G. B. Suffritti, The behaviour of water confined in zeolites: molecular dynamics simulations versus experiment, J. Phys. Condens. Matter 22(28), 284106 (2010)
P. Cicu, P. Demontis, S. Spanu, G. B. Suffritti, and A. Tilocca, Electric-field-dependent empirical potentials for molecules and crystals: A first application to flexible water molecule adsorbed in zeolites, J. Chem. Phys. 112(19), 8267 (2000)
S. Izadi, R. Anandakrishnan, and A. V. Onufriev, Building water models: A different approach, J. Phys. Chem. Lett. 5(21), 3863 (2014)
R. Anandakrishnan, C. Baker, S. Izadi, and A. V. Onufriev, Point charges optimally placed to represent the multipole expansion of charge distributions, PLoS ONE 8, e67715 (2013)
C. Bergonzo and T. E. Cheatham, Improved force field parameters lead to a better description of RNA structure, J. Chem. Theory Comput. 11 (9), 3969 (2015)
K. Gao, J. Yin, N. M. Henriksen, A. T. Fenley, and M. K. Gilson, Binding enthalpy calculations for a neutral hostguest pair yield widely divergent salt effects across water models, J. Chem. Theory Comput. 11(10), 4555 (2015)
C. N. Nguyen, T. Kurtzman, and M. K. Gilson, Spatial decomposition of translational water-water correlation entropy in binding pockets, J. Chem. Theory Comput. 12(1), 414 (2016)
F. Häse and M. Zacharias, Free energy analysis and mechanism of base pair stacking in nicked DNA, Nucleic Acids Res. 44 (15), 7100 (2016)
A. Mukhopadhyay, I. S. Tolokh, and A. V. Onufriev, Accurate evaluation of charge asymmetry in aqueous solvation, J. Phys. Chem. B 119(20), 6092 (2015)
J. C. Phillips, R. Braun, W. Wang, J. Gumbart, E. Tajkhorshid, E. Villa, C. Chipot, R. D. Skeel, L. Kale, and K. Schulten, Scalable molecular dynamics with NAMD, J. Comput. Chem. 26(16), 1781 (2005)
I. C. Yeh and G. Hummer, System-size dependence of diffusion coefficients and viscosities from molecular dynamics simulations with periodic boundary conditions, J. Phys. Chem. B 108(40), 15873 (2004)
R. G. Gordon, Advances in Magnetic Resonance, Vol. 3, p. 1, New York: Academic Press Inc., 1968
A. Y. Zasetsky, Dielectric relaxation in liquid water: Two fractions or two dynamics? Phys. Rev. Lett. 107 (11), 117601 (2011)
C. J. Fecko, J. J. Loparo, S. T. Roberts, and A. Tokmakoff, Local hydrogen bonding dynamics and collective reorganization in water: Ultrafast infrared spectroscopy of HOD/D2O, J. Chem. Phys. 122(5), 054506 (2005)
J. J. Loparo, S. T. Roberts, and A. Tokmakoff, Multidimensional infrared spectroscopy of water (I): Vibrational dynamics in two-dimensional IR line shapes, J. Chem. Phys. 125(19), 194521 (2006)
J. J. Loparo, S. T. Roberts, and A. Tokmakoff, Multidimensional infrared spectroscopy of water (II): Hydrogen bond switching dynamics, J. Chem. Phys. 125(19), 194522 (2006)
J. Stenger, D. Madsen, P. Hamm, E. T. Nibbering, and T. Elsaesser, A photon echo peak shift study of liquid water, J. Phys. Chem. A 106 (10), 2341 (2002)
M. Cowan, B. D. Bruner, N. Huse, J. Dwyer, B. Chugh, E. Nibbering, T. Elsaesser, and R. Miller, Ultrafast memory loss and energy redistribution in the hydrogen bond network of liquid H2O, Nature 434 (7030), 199 (2005)
A. Luzar and D. Chandler, Hydrogen-bond kinetics in liquid water, Nature 379 (6560), 55 (1996)
A. Luzar and D. Chandler, Effect of environment on hydrogen bond dynamics in liquid water, Phys. Rev. Lett. 76(6), 928 (1996)
F. W. Starr, J. K. Nielsen, and H. E. Stanley, Fast and slow dynamics of hydrogen bonds in liquid water, Phys. Rev. Lett. 82(11), 2294 (1999)
F. W. Starr, J. K. Nielsen, and H. E. Stanley, Hydrogenbond dynamics for the extended simple point-charge model of water, Phys. Rev. E 62(1), 579 (2000)
A. Luzar, Resolving the hydrogen bond dynamics conundrum, J. Chem. Phys. 113(23), 10663 (2000)
A. Luzar, Extent of inter-hydrogen bond correlations in water: Temperature effect, Chem. Phys. 258(2–3), 267 (2000)
V. Voloshin and Y. I. Naberukhin, Hydrogen bond lifetime distributions in computer simulated water, J. Struct. Chem. 50(1), 78 (2009)
H. Martiniano and N. Galamba, Insights on hydrogenbond lifetimes in liquid and supercooled water, J. Phys. Chem. B 117(50), 16188 (2013)
B. Mukherjee, Microscopic origin of temporal heterogeneities in translational dynamics of liquid water, J. Chem. Phys. 143(5), 054503 (2015)
O. Conde and J. Teixeira, Hydrogen bond dynamics in water studied by depolarized Rayleigh scattering, J. Phys. 44(4), 525 (1983)
J. Teixeira, M. C. Bellissent-Funel, S. H. Chen, and A. J. Dianoux, Experimental determination of the nature of diffusive motions of water molecules at low temperatures, Phys. Rev. A 31(3), 1913 (1985)
C. Fecko, J. Eaves, J. Loparo, A. Tokmakoff, and P. Geissler, Ultrafast hydrogen-bond dynamics in the infrared spectroscopy of water, Science 301 (5640), 1698 (2003)
D. Laage, Reinterpretation of the liquid water quasielastic neutron scattering spectra based on a nondiffusive jump reorientation mechanism, J. Phys. Chem. B 113(9), 2684 (2009)
R. Kumar, J. Schmidt, and J. Skinner, Hydrogen bonding definitions and dynamics in liquid water, J. Chem. Phys. 126(20), 204107 (2007)
D. Prada-Gracia, R. Shevchuk, and F. Rao, The quest for self-consistency in hydrogen bond definitions, J. Chem. Phys. 139(8), 084501 (2013)
A. Ozkanlar, T. Zhou, and A. E. Clark, Towards a unified description of the hydrogen bond network of liquid water: A dynamics based approach, J. Chem. Phys. 141(21), 214107 (2014)
P. Wernet, D. Nordlund, U. Bergmann, M. Cavalleri, M. Odelius, H. Ogasawara, L. Å. Näslund, T. K. Hirsch, L. Ojamäe, P. Glatzel, L. G. M. Pettersson, and A. Nilsson, The structure of the first coordination shell in liquid water, Science 304 (5673), 995 (2004)
R. H. Henchman and S. J. Irudayam, Topological hydrogen-bond definition to characterize the structure and dynamics of liquid water, J. Phys. Chem. B 114(50), 16792 (2010)
J. Jonas, T. DeFries, and D. Wilbur, Molecular motions in compressed liquid water, J. Chem. Phys. 65(2), 582 (1976)
J. Ropp, C. Lawrence, T. Farrar, and J. Skinner, Rotational motion in liquid water is anisotropic: a nuclear magnetic resonance and molecular dynamics simulation study, J. Am. Chem. Soc. 123(33), 8047 (2001)
E. H. Hardy, A. Zygar, M. D. Zeidler, M. Holz, and F. D. Sacher, Isotope effect on the translational and rotational motion in liquid water and ammonia, J. Chem. Phys. 114(7), 3174 (2001)
R. Ludwig, F. Weinhold, and T. C. Farrar, Experimental and theoretical determination of the temperature dependence of deuteron and oxygen quadrupole coupling constants of liquid water, J. Chem. Phys. 103(16), 6941 (1995)
J. A. Sellberg, C. Huang, T. A. McQueen, N. D. Loh, H. Laksmono, et al., Ultrafast X-ray probing of water structure below the homogeneous ice nucleation temperature, Nature 510 (7505), 381 (2014)
C. Angell and F. Franks, Water: A Comprehensive Treatise, Vol. 7, New York: Plenum, 1982
H. E. Stanley and O. Mishima, The relationship between liquid, supercooled and glassy water, Nature 396 (6709), 329 (1998)
L. Liu, S. H. Chen, A. Faraone, C. W. Yen, and C. Y. Mou, Pressure dependence of fragile-to-strong transition and a possible second critical point in supercooled confined water, Phys. Rev. Lett. 95(11), 117802 (2005)
S. V. Lishchuk, N. P. Malomuzh, and P. V. Makhlaichuk, Why thermodynamic properties of normal and heavy water are similar to those of argon-like liquids? Phys. Lett. A 374(19–20), 2084 (2010)
A. Fisenko, N. Malomuzh, and A. Oleynik, To what extent are thermodynamic properties of water argonlike? Chem. Phys. Lett. 450(4–6), 297 (2008)
S. Izadi, B. Aguilar, and A. V. Onufriev, Proteinligand electrostatic binding free energies from explicit and implicit solvation, J. Chem. Theory Comput. 11(9), 4450 (2015)
D. Nayar and C. Chakravarty, Sensitivity of local hydration behaviour and conformational preferences of peptides to choice of water model, Phys. Chem. Chem. Phys. 16(21), 10199 (2014)
R. B. Best and J. Mittal, Protein simulations with an optimized water model: Cooperative helix formation and temperature-induced unfolded state collapse, J. Phys. Chem. B 114(46), 14916 (2010)
R. B. Best and J. Mittal, Free-energy landscape of the gb1 hairpin in all-atom explicit solvent simulations with different force fields: Similarities and differences, Proteins 79(4), 1318 (2011)
P. Florová, P. Sklenovský, P. Banáš, and M. Otyepka, Explicit water models affect the specific solvation and dynamics of unfolded peptides while the conformational behavior and flexibility of folded peptides remain intact, J. Chem. Theory Comput. 6(11), 3569 (2010)
H. E. Stanley, S. V. Buldyrev, G. Franzese, N. Giovambattista, and F. W. Starr, Static and dynamic heterogeneities in water, Philosophical Transactions of the Royal Society of London A 363 (1827), 509 (2005)
A. Nilsson and L. Pettersson, Perspective on the structure of liquid water, Chem. Phys. 389(1–3), 1 (2011)
D. Prada-Gracia, R. Shevchuk, P. Hamm, and F. Rao, Towards a microscopic description of the free-energy landscape of water, J. Chem. Phys. 137(14), 144504 (2012)
G. C. Picasso, D. C. Malaspina, M. A. Carignano, and I. Szleifer, Cooperative dynamic and diffusion behavior above and below the dynamical crossover of supercooled water, J. Chem. Phys. 139(4), 044509 (2013)
J. A. Sellberg, S. Kaya, V. H. Segtnan, C. Chen, T. Tyliszczak, H. Ogasawara, D. Nordlund, L. G. M. Pettersson, and A. Nilsson, Comparison of X-ray absorption spectra between water and ice: New ice data with low pre-edge absorption cross-section, J. Chem. Phys. 141(3), 034507 (2014)
E. Duboué-Dijon and D. Laage, Characterization of the local structure in liquid water by various order parameters, J. Phys. Chem. B 119(26), 8406 (2015)
R. S. Singh, J. W. Biddle, P. G. Debenedetti, and M. A. Anisimov, Two-state thermodynamics and the possibility of a liquid-liquid phase transition in supercooled TIP4P/2005 water, J. Chem. Phys. 144(14), 144504 (2016)
Y. Xu, N. G. Petrik, R. S. Smith, B. D. Kay, and G. A. Kimmel, Growth rate of crystalline ice and the diffusivity of supercooled water from 126 to 262 K, Proc. Natl. Acad. Sci. USA 113(52), 14921 (2016)
K. A. Jackson, Kinetic Processes: Crystal Growth, Diffusion, and Phase Transitions in Materials, Wiley-VCH Verlag GmbH & Co. KGaA, 2005
W. L. Jorgensen, J. Chandrasekhar, J. D. Madura, R. W. Impey, and M. L. Klein, Comparison of simple potential functions for simulating liquid water, J. Chem. Phys. 79(2), 926 (1983)
J. L. F. Abascal and C. Vega, A general purpose model for the condensed phases of water: TIP4P/2005, J. Chem. Phys. 123 (23), 234505 (2005)
M. D. Marzio, G. Camisasca, M. Rovere, and P. Gallo, Fragile-to-strong crossover in supercooled water: A comparison between TIP4P and TIP4P/2005 models, Nuovo Cim. 39(C), 302 (2016)
Acknowledgements
This research was supported by the Italian Ministero dell’Istruzione, dell’Università, e della Ricerca (MIUR), by Regione Autonoma della Sardegna (Italy), by Università degli studi di Sassari, and by Istituto Nazionale per la Scienza e Tecnologia dei Materiali (INSTM), which we acknowledge. The “Consorzio COSMOLAB” is also acknowledged for the resources provided within the CyberSar Project. A.V.O. acknowledges support from the US National Institutes of Health (NIH GM076121). We are grateful to Professor G. Franzese for useful discussion of our results and for encouragement in continuing our study. Professor F. Mallamace is gratefully acknowledged for critically reading the manuscript and making useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gabrieli, A., Sant, M., Izadi, S. et al. High-temperature dynamic behavior in bulk liquid water: A molecular dynamics simulation study using the OPC and TIP4P-Ew potentials. Front. Phys. 13, 138203 (2018). https://doi.org/10.1007/s11467-017-0693-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11467-017-0693-7