Abstract
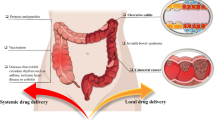
Colon-targeted oral delivery is crucial for the treatment of colon-related diseases, as this delivery strategy enables precise drug administration to the diseased site, enhances drug bioavailability, and improves patient compliance. In particular, nanoparticle-based oral formulations shield drugs from the harsh gastrointestinal environment, and selectively increase drug concentration inside diseased colon cells, thus elevating therapeutic efficacy while reducing systemic toxicity. In this review, we elaborate recent progress in this area, with emphasis on the pathophysiological characteristics of colon site and design strategies to take advantage of these characteristics for colon targeting.
摘要
设计口服结肠靶向的递送系统对于治疗结肠有关的疾病非常重要,因为这种方式能够使药物精确施用到病变部位,提高药物生物利用度和患者的顺应性。尤其,以纳米粒子为基础的口服制剂可以保护药物在恶劣的胃肠道环境中不被破坏,并选择性地增加病变结肠部位的药物浓度,从而提高治疗功效、降低全身毒性。本文阐述了这方面的最新进展,重点总结了结肠部位的病生理特征和以此为依据的靶向设计策略。







Similar content being viewed by others
References
Amidon S, Brown JE, Dave VS (2015) Colon-targeted oral drug delivery systems: design trends and approaches. AAPS PharmSciTech 16:731–741
Pinto JF (2010) Site-specific drug delivery systems within the gastro-intestinal tract: from the mouth to the colon. Int J Pharm 395:44–52
Rodriguez M, Antunez JA, Taboada C et al (2001) Colon-specific delivery of budesonide from microencapsulated cellulosic cores: evaluation of the efficacy against colonic inflammation in rats. J Pharm Pharmacol 53:1207–1215
Dressman JB, Berardi RR, Dermentzoglou LC et al (1990) Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res 7:756–761
Cook MT, Tzortzis G, Charalampopoulos D et al (2012) Microencapsulation of probiotics for gastrointestinal delivery. J Control Release 162:56–67
Pullan RD, Thomas GA, Rhodes M et al (1994) Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 35:353–359
Lamprecht A, Yamamoto H, Takeuchi H et al (2005) Nanoparticles enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats. J Pharmacol Exp Ther 315:196–202
Li JX (2015) Nanotechnology-based platform for early diagnosis of cancer. Sci Bull 60:488–490
Meng H, Wei R (2015) Use of smart designed nanoparticles to impact cancer surgery. Sci Bull 60:142–143
Wang C (2015) Bio-cluster nano-bomb for cancer drug delivery: efficacious fire at the target. Sci Bull 60:403–404
Zhao F, Meng H, Yan L et al (2015) Nanosurface chemistry and dose govern the bioaccumulation and toxicity of carbon nanotubes, metal nanomaterials and quantum dots in vivo. Sci Bull 60:3–20
Zhao RF, Wang H, Ji TJ et al (2015) Biodegradable cationic epsilon-poly-L-lysine-conjugated polymeric nanoparticles as a new effective antibacterial agent. Sci Bull 60:216–226
Lamprecht A (2010) IBD: selective nanoparticle adhesion can enhance colitis therapy. Nat Rev Gastroenterol hepatol 7:311–312
Amekyeh H, Billa N, Yuen KH et al (2015) A gastrointestinal transit study on amphotericin B-loaded solid lipid nanoparticles in rats. AAPS PharmSciTech 16:871–877
El-Zawawy LA, El-Said D, Mossallam SF et al (2015) Triclosan and triclosan-loaded liposomal nanoparticles in the treatment of acute experimental toxoplasmosis. Exp Parasitol 149:54–64
Xiao B, Merlin D (2012) Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease. Expert Opin Drug Deliv 9:1393–1407
Guo F, Zhang M, Gao Y et al (2015) Modified nanoparticles with cell-penetrating peptide and amphipathic chitosan derivative for enhanced oral colon absorption of insulin: preparation and evaluation. Drug Deliv. doi:10.3109/10717544.2015.1048489
Yang Y, Xie XY, Mei XG (2016) Preparation and in vitro evaluation of thienorphine-loaded PLGA nanoparticles. Drug Deliv 23:787–793
Joshi G, Kumar A, Sawant K (2014) Enhanced bioavailability and intestinal uptake of Gemcitabine HCl loaded PLGA nanoparticles after oral delivery. Eur J Pharm Sci 60:80–89
Hossain MA, Yamashita M, Vong LB et al (2014) Silica-installed redox nanoparticles for novel oral nanotherapeutics—improvement in intestinal delivery with anti-inflammatory effects. J Drug Target 22:638–647
Zhao Z, Gao Y, Wu C et al (2016) Development of novel core-shell dual-mesoporous silica nanoparticles for the production of high bioavailable controlled-release fenofibrate tablets. Drug Dev Ind Pharm 42:199–208
Bhavsar MD, Amiji MM (2007) Gastrointestinal distribution and in vivo gene transfection studies with nanoparticles-in-microsphere oral system (NiMOS). J Control Release 119:339–348
Bhavsar MD, Amiji MM (2008) Oral IL-10 gene delivery in a microsphere-based formulation for local transfection and therapeutic efficacy in inflammatory bowel disease. Gene Ther 15:1200–1209
Bhavsar MD, Amiji MM (2008) Development of novel biodegradable polymeric nanoparticles-in-microsphere formulation for local plasmid DNA delivery in the gastrointestinal tract. AAPS PharmSciTech 9:288–294
Laroui H, Dalmasso G, Nguyen HT et al (2010) Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology 138(843–853):e841–e842
Laroui H, Theiss AL, Yan Y et al (2011) Functional TNFalpha gene silencing mediated by polyethyleneimine/TNFalpha siRNA nanocomplexes in inflamed colon. Biomaterials 32:1218–1228
Theiss AL, Laroui H, Obertone TS et al (2011) Nanoparticle-based therapeutic delivery of prohibitin to the colonic epithelial cells ameliorates acute murine colitis. Inflamm Bowel Dis 17:1163–1176
Sun S, Liang N, Yamamoto H et al (2015) pH-sensitive poly(lactide-co-glycolide) nanoparticle composite microcapsules for oral delivery of insulin. Int J Nanomed 10:3489–3498
Makhlof A, Tozuka Y, Takeuchi H (2009) pH-sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur J Pharm Biopharm 72:1–8
Prasad S, Dangi JS (2015) Development and characterization of pH responsive polymeric nanoparticles of SN-38 for colon cancer. Artif Cells Nanomed Biotechnol. doi:10.3109/21691401.2015.1105239
Araujo F, Shrestha N, Shahbazi MA et al (2015) Microfluidic assembly of a multifunctional tailorable composite system designed for site specific combined oral delivery of peptide drugs. ACS Nano 9:8291–8302
Lai SK, Wang YY, Hanes J (2009) Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev 61:158–171
Antoni L, Nuding S, Wehkamp J et al (2014) Intestinal barrier in inflammatory bowel disease. World J Gastroenterol 20:1165–1179
Collnot EM, Ali H, Lehr CM (2012) Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release 161:235–246
Niebel W, Walkenbach K, Beduneau A et al (2012) Nanoparticle-based clodronate delivery mitigates murine experimental colitis. J Control Release 160:659–665
Jubeh TT, Barenholz Y, Rubinstein A (2004) Differential adhesion of normal and inflamed rat colonic mucosa by charged liposomes. Pharma Res 21:447–453
Beloqui A, Coco R, Alhouayek M et al (2013) Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. Int J Pharm 454:775–783
Wang YY, Lai SK, Suk JS et al (2008) Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew Chem Int Ed 47:9726–9729
Lautenschlager C, Schmidt C, Lehr CM et al (2013) PEG-functionalized microparticles selectively target inflamed mucosa in inflammatory bowel disease. Eur J Pharm Biopharm 85:578–586
Wilcox MD, Van Rooij LK, Chater PI et al (2015) The effect of nanoparticle permeation on the bulk rheological properties of mucus from the small intestine. Eur J Pharm Biopharm 96:484–487
Shan W, Zhu X, Liu M et al (2015) Overcoming the diffusion barrier of mucus and absorption barrier of epithelium by self-assembled nanoparticles for oral delivery of insulin. ACS Nano 9:2345–2356
Akande J, Yeboah KG, Addo RT et al (2010) Targeted delivery of antigens to the gut-associated lymphoid tissues: 2. Ex vivo evaluation of lectin-labelled albumin microspheres for targeted delivery of antigens to the M-cells of the Peyer’s patches. J Microencapsul 27:325–336
Anosova NG, Chabot S, Shreedhar V et al (2008) Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer’s patches. Mucosal Immunol 1:59–67
Borges O, Cordeiro-da-Silva A, Romeijn SG et al (2006) Uptake studies in rat Peyer’s patches, cytotoxicity and release studies of alginate coated chitosan nanoparticles for mucosal vaccination. J Control Release 114:348–358
Chen H, Torchilin V, Langer R (1996) Lectin-bearing polymerized liposomes as potential oral vaccine carriers. Pharm Res 13:1378–1383
Brandtzaeg P (2009) Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol 70:505–515
D’Souza B, Bhowmik T, Shashidharamurthy R et al (2012) Oral microparticulate vaccine for melanoma using M-cell targeting. J Drug Target 20:166–173
Simmonds NJ, Allen RE, Stevens TR et al (1992) Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology 103:186–196
Wilson DS, Dalmasso G, Wang L et al (2010) Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. NatMater 9:923–928
Vong LB, Tomita T, Yoshitomi T et al (2012) An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology 143:1027–1036
Xiao B, Laroui H, Ayyadurai S et al (2013) Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-alpha RNA interference for IBD therapy. Biomaterials 34:7471–7482
Laroui H, Viennois E, Bo X et al (2014) Fab′-bearing siRNA TNFα-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J Control Release 186:41–53
Xiao B, Laroui H, Viennois E et al (2014) Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology 146:1289–1300
Izadi Z, Divsalar A, Saboury AA et al (2016) β-lactoglobulin-pectin nanoparticle-based oral drug delivery system for potential treatment of colon cancer. Chem Biol Drug Design. doi:10.1111/cbdd.12748
Wang Y, Qin F, Tan H et al (2015) pH-responsive glycol chitosan-cross-linked carboxymethyl-beta-cyclodextrin nanoparticles for controlled release of anticancer drugs. Int J Nanomed 10:7359–7369
Gupta PN, Kapil K, Goyal AK et al (2007) M-cell targeted biodegradable PLGA nanoparticles for oral immunization against hepatitis B. J Drug Target 15:701–713
Ma T, Wang L, Yang T et al (2014) Homogeneous PLGA-lipid nanoparticle as a promising oral vaccine delivery system for ovalbumin. Asian J Pharm Sci 9:129–136
Lai LM, Zhao CQ, Su MN et al (2015) In vivo rapid fluorescence imaging of Alzheimer’s disease through accurate target bio-marking of zinc gluconate. Sci Bull 60:1465–1467
Zhang WB, Gao CY (2015) Recent advances in cell imaging and cytotoxicity of intracellular stimuli-responsive nanomaterials. Sci Bull 60:1973–1979
Zhao YL (2015) Sensing system for mimicking cancer cell-drug interaction. Sci Bull 60:1218–1219
Rhee JK, Park OK, Lee A et al (2014) Glycol chitosan-based fluorescent theranostic nanoagents for cancer therapy. Mar Drugs 12:6038–6057
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81471779), the National Thousand Young Talents Program, and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lei Lu and Gaoxian Chen contributed equally to this work.
About this article
Cite this article
Lu, L., Chen, G., Qiu, Y. et al. Nanoparticle-based oral delivery systems for colon targeting: principles and design strategies. Sci. Bull. 61, 670–681 (2016). https://doi.org/10.1007/s11434-016-1056-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-016-1056-4