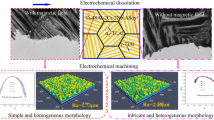
Abstract
Electrochemical machining (ECM) is becoming increasingly important for the efficient machining of parts with a large machining area. This is an addition challenge for ECM because of the very high machining current. To overcome this difficulty, a direct and effective strategy is to adopt the machining mode that uses a low-concentration electrolyte with a low current density. The purpose of this study is to reveal the electrochemical behaviour and surface morphology in low-concentration electrolyte. The polarization behavior of Ti-48Al-2Cr-2Nb is measured by linear sweep voltammetry and cyclic voltammetry curves. The ηω-j curves demonstrate the special dissolution behaviour of Ti-48Al-2Cr-2Nb at low current densities. The surface morphology, surface quality, and dissolution mechanism are analysed in three low-concentration electrolytes at different current densities after the ECM dissolution experiments. The results demonstrate that Ti-48Al-2Cr-2Nb exhibits three unique dissolution morphologies in the three solutions, and we found that the γ-TiAl phase dissolves faster than the α2-Ti3Al phase. These results also show that 1% NaCl solution is more suitable for Ti-48Al-2Cr-2Nb in ECM compared with the other two solutions, considering its good surface quality, low breakdown potential, and high material removal rate. Later, the dissolution process of the sample in 1% NaCl solution at different corrosion times is revealed. Moreover, a dissolution model is proposed for the electrochemical dissolution behaviour of Ti-48Al-2Cr-2Nb in 1% NaCl solution.
Similar content being viewed by others
References
Rajurkar K P, Zhu D, McGeough J A, et al. New developments in electro-chemical machining. CIRP Ann, 1999, 48: 567–579
Xu Z, Xu Q, Zhu D, et al. A high efficiency electrochemical machining method of blisk channels. CIRP Ann, 2013, 62: 187–190
Xu Z, Wang Y. Electrochemical machining of complex components of aero-engines: Developments, trends, and technological advances. Chin J Aeronaut, 2019, doi: 10.1016/j.cja.2019.09.016
Klocke F, Klink A, Veselovac D, et al. Turbomachinery component manufacture by application of electrochemical, electro-physical and photonic processes. CIRP Ann, 2014, 63: 703–726
Fang X, Qu N, Zhang Y, et al. Improvement of hole exit accuracy in electrochemical drilling by applying a potential difference between an auxiliary electrode and the anode. J Mater Process Tech, 2014, 214: 556–564
Gu Z, Zhu D, Xue T, et al. Investigation on flow field in electrochemical trepanning of aero engine diffuser. Int J Adv Manuf Technol, 2017, 89: 877–884
Guo P, Lin X, Li J, et al. Electrochemical behavior of Inconel 718 fabricated by laser solid forming on different sections. Corrosion Sci, 2018, 132: 79–89
Guo P, Lin X, Xu J, et al. Electrochemical removal of different phases from laser solid formed Inconel 718. J Electrochem Soc, 2017, 164: E151–E157
Wang D, Zhu Z, Wang N, et al. Investigation of the electrochemical dissolution behavior of Inconel 718 and 304 stainless steel at low current density in NaNO3 solution. Electrochim Acta, 2015, 156: 301–307
Ge Y, Zhu Z, Wang D. Electrochemical dissolution behavior of the nickel-based cast superalloy K423A in NaNO3 solution. Electrochim Acta, 2017, 253: 379–389
Xu Z, Chen X, Zhou Z, et al. Electrochemical machining of hightemperature titanium alloy Ti60. Procedia CIRP, 2016, 42: 125–130
Wang Y, Xu Z, Zhang A. Electrochemical dissolution behavior of Ti-45Al-2Mn-2Nb+0.8 vol% TiB2 XD alloy in NaCl and NaNO3 solutions. Corrosion Sci, 2019, 157: 357–369
Wang Y, Xu Z, Zhang A. Comparison of the electrochemical dissolution behavior of extruded and casted Ti-48Al-2Cr-2Nb alloys in NaNO3 solution. J Electrochem Soc, 2019, 166: E347–E357
Sharman A R C, Aspinwall D K, Dewes R C, et al. The effects of machined workpiece surface integrity on the fatigue life of γ-titanium aluminide. Int J Machine Tools Manuf, 2001, 41: 1681–1685
Liu J, Zhu D, Zhao L, et al. Experimental investigation on electrochemical machining of γ-TiAl intermetallic. Procedia CIRP, 2015, 35: 20–24
Klocke F, Herrig T, Zeis M, et al. Experimental research on the electrochemical machinability of selected γ-TiAl alloys for the manufacture of future aero engine components. Procedia CIRP, 2015, 35: 50–54
Wang Y, Xu Z, Zhang A. Anodic characteristics and electrochemical machining of two typical γ-TiAl alloys and its quantitative dissolution model in NaNO3 solution. Electrochim Acta, 2020, 331: 135429
Kothari K, Radhakrishnan R, Wereley N M. Advances in gamma titanium aluminides and their manufacturing techniques. Prog Aerosp Sci, 2012, 55: 1–16
Aspinwall D K, Dewes R C, Mantle A L. The machining of γ-TiAl intermetallic alloys. CIRP Ann, 2005, 54: 99–104
Klocke F, Settineri L, Lung D, et al. High performance cutting of gamma titanium aluminides: Influence of lubricoolant strategy on tool wear and surface integrity. Wear, 2013, 302: 1136–1144
Hood R, Aspinwall D K, Soo S L, et al. Workpiece surface integrity when slot milling γ-TiAl intermetallic alloy. CIRP Ann, 2014, 63: 53–56
Bewlay B P, Weimer M, Kelly T, et al. The science, technology, and implementation of TiAl alloys in commercial aircraft engines. MRS Proc, 2013, 1516: 49–58
Ittah R, Amsellem E, Itzhak D. Pitting corrosion evaluation of titanium in NH4Br solutions by electrochemical methods. Int J Electrochem Sci, 2014, 9: 633–643
Klocke F, Zeis M, Klink A, et al. Experimental research on the electrochemical machining of modern titanium- and nickel-based alloys for aero engine components. Procedia CIRP, 2013, 6: 368–372
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China (Grant No. 91960204), the Natural Science Foundation for Distinguished Young Scholars of Jiangsu Province (Grant No. BK20170031), and the Fundamental Research Funds for the Central Universities (Grant No. NE2014104).
Rights and permissions
About this article
Cite this article
Wang, Y., Xu, Z., Zhang, A. et al. Surface morphology and electrochemical behaviour of Ti-48Al-2Cr-2Nb alloy in low-concentration salt solution. Sci. China Technol. Sci. 64, 283–296 (2021). https://doi.org/10.1007/s11431-019-1558-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-019-1558-8