Abstract
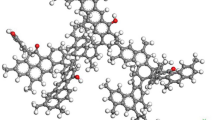
Clarification of the molecular mechanism underlying the interaction of coal with CH4, CO2, and H2O molecules is the basis for an in-depth understanding of the states of fluid in coal and fluid-induced coal swelling/contraction. In terms of instrumental analysis, molecular simulation technology based on molecular mechanics/dynamics and quantum chemistry is a powerful tool for revealing the relationship between the structure and properties of a substance and understanding the interaction mechanisms of physical-chemical systems. In this study, the giant canonical ensemble Monte Carlo (GCMC) and molecular dynamics (MD) methods were applied to investigate the adsorption behavior of a Yanzhou coal model (C222H185N3O17S5). We explored the adsorption amounts of CH4, CO2, and H2O onto Yanzhou coal, the adsorption conformation, and the impact of oxygen-containing functional groups. Furthermore, we revealed the different adsorption mechanisms of the three substances using isosteric heat of adsorption and energy change data. (1) The adsorption isotherms of the mono-component CH4, CO2, and H2O were consistent with the Langmuir model, and their adsorption amounts showed an order of CH4<CO2<H2O. In addition, high temperatures were non-conducive to adsorption. When the three components of CH4/CO2/H2O were mixed (at a molar ratio of 1:1:1) for adsorption, only the adsorption curve of H2O was consistent with the Langmuir model. (2) The mean values of the isosteric heat of adsorption of CH4, CO2, and H2O were 22.54, 36.90, and 37.82 kJ/mol, respectively; that is, H2O>CO2>CH4. In addition, at higher temperatures, the isosteric heat of adsorption decreased; pressure had no significant effect on the heat of adsorption. (3) CH4 molecules displayed an aggregated distribution in the pores, whereas CO2 molecules were cross arranged in pairs. Regarding H2O molecules, under the influence of hydrogen bonds, the O atom pointed to surrounding H2O molecules or the H atoms of coal molecules in a regular pattern. The intermolecular distances of the three substances were 0.421, 0.553, and 0.290 nm, respectively. The radial distribution function (RDF) analysis showed that H2O molecules were arranged in the most compact fashion, forming a tight molecular layer. (4) H2O molecules showed a significantly stratified distribution around oxygen-containing functional groups on the coal surface, and the bonding strength showed a descending order of hydroxyl> carboxyl>carbonyl. In contrast, CO2 and CH4 showed only slightly stratified distributions. (5) After the adsorption of CH4, CO2, and H2O, the total energy, the energy of valence electrons, and the non-bonding interaction of the system in the Yanzhou coal model all decreased. The results regarding the decrease in the total energy of the system indicated an order of H2O>CO2>CH4 in terms of the adsorption priority of the Yanzhou coal model. The results regarding the decrease in the energy of valence electrons showed that under certain geological conditions, a pressure-induced “coal strain” could lead to a structural rearrangement during the interaction of coal with fluid to form a more stable conformation, which might be the molecular mechanism of coal swelling resulting from the interaction between fluid and coal. An analysis of the contribution of Van der Waals forces, electrostatic interactions and hydrogen bonds to the decrease in non-bonding interactions revealed the mechanism underlying the interactions between coal molecules and the three substances. The interaction between coal molecules and CH4 consisted of typical physical adsorption, whereas that between coal molecules and CO2 consisted mainly of physical adsorption combined with weak chemical adsorption. The interaction between coal molecules and H2O is physical and chemical.
Similar content being viewed by others
References
Astashov A V, Belyi A A, Bunin A V. 2008. Quasi-equilibrium swelling and structural parameters of coals. Fuel, 87: 3455–3461
Brochard L, Vandamme M, Pellenq R J M, et al. 2012. Adsorption-induced deformation of microporous materials coal. Langmuir, 28: 2659–2670
Busch A, Gensterblum Y, Krooss B M. 2003. Methane and CO2 sorption and desorption measurements on dry Argonne premium coals: Pure components and mixtures. Int J Coal Geol, 55: 205–224
Busch A, Gensterblum Y, Krooss B M, et al. 2006. Investigation of high pressure selective adsorption/desorption CO2 and CH4 on coals: An experimental study. Int J Coal Geol, 66: 53–68
Bustin R M, Clarkson C R. 1998. Geological controls on coalbed methane reservoir capacity and gas content. Int J Coal Geol, 38: 3–26
Cao D P, Gao G T, Wang W C. 2000. Grand canonical ensemble monte carlo simulation of adsorption storage of methane in slit micropores (in Chinese). J Chem Ind Eng, 1: 23–30
Carlson G A. 1992. Computer simulation of the molecular structure of bituminous coal. Energy Fuels, 6: 771–778
Chen C G, Wei X W, Xian X F. 2000. AB intio study on the interaction between CH4 and the coal surface (in Chinese). J Chongqing Univ, 23: 77–83
Chen Z L, Xu W R, Tang L D. 2007. The Theory and Practice of Molecular Simulation (in Chinese). Beijing: Chemical Industry Press. 79–80
Cui Y J, Zhang Q L, Yang X L. 2003. Changes of adsorption capability and isosteric heat of different coal (in Chinese). Nat Gas Ind, 23: 130–131
Cui Y J, Zhang Q, Zhang H, et al. 2005. Adsorption of different rank coals to single component gases (in Chinese). Nat Gas Ind, 25: 61–65
Dai S F, Zhang B B, Zhu C S, et al. 2009. Isothermal adsorption of CH4/CO2 mixed gas for the late Paleozoic coals from the Kailuan coalfield of Hebei province (in Chinese). J China Coal Soc, 34: 578–583
Day S, Sakurovs z, Weir S. 2008. Supercritical gas sorption on moist coals. Int J Coal Geol, 74: 203–214
Einstein A. 1905. On the movement of small particles suspend edina stationary liquid demanded by the molecular-kinetic theory of heat. Ann Phys (Leipzig), 17: 549–560
Ewald P P. 1921. Die berechnung optischer und elekrostatischer gitterpotentiale. Ann Phys, 369: 253–287
Fu X C, Shen W X, Yao T Y. 1993. Physical Chemistry. Vol.2 (in Chinese). Beijing: Higher Education Press. 950
Goodman A L, Campus L M, Schroeder K T. 2005. Direct evidence of carbon dioxide sorption on Argonne premium coals using attenuated total reflectance-fourier transform infrared spectroscopy. Energy Fuels, 19: 471–476
Goodman A L, Favors R N, Larsen J W. 2006. Argonne coal structure rearrangement caused by sorption of CO2. Energy Fuels, 20: 2537–2543
Goodman A L. 2009. A comparison study of carbon dioxide adsorption on polydimethylsiloxane, silica gel, and Illinois no. 6 coal using in situ infrared spectroscopy. Energy Fuels, 23: 1101–1106
Hu H X, Li X C, Fang Z M. 2010. Small-molecule gas sorption and diffusion in coal molecular simulation. Energy, 35: 2939–2944
Jhon Y H, Cho M, Jeon H R, et al. 2007. Simulation of methane adsorption and diffusion within alkoxy-functionalized IRMOFs exhibiting severely disordered crystal structure. J Phys Chem C, 111: 16618–16625
Jing W. 2007. Molecular simulation of adsorption and diffusion of methane in deformed coal (in Chinese). Dissertation for Master Degree. Taiyuan: Taiyuan University of Technology
Karasawa N, Goddard W A. 1992. Force fields, structures, and properties of polyvinylidene fluoride crystal. Macromolecules, 25: 7268–7281
Krooss B M, van Bergen F, Gensterblum Y, et al. 2002. High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. Int J Coal Geol, 51: 69–91
Larsen J W, Flowers R A, Hall P J, et al. 1997. Structural rearrangement of strained coals. Energy Fuels, 11: 998–1002
Larsen J W. 2004. The effects of dissolved CO2 on coal structure and properties. Int J Coal Geo, 57: 63–70
Levy J H, Day S J, Killingley J S. 1997. Methane capacities of Bowen Basin coals related to coal properties. Fuel, 76: 813–819
Lin J T, Guo Y Y, Wu S Y. 2001. Sorption of coal to different gases in the course of coalbed methane exploited through injection of another gas (in Chinese). J Taiyuan Univ Technol, 32: 18–20
Liu Y Y, Wilcox J. 2012. Effects of surface heterogeneity on the adsorption of CO2 in microporous carbons. Environ Sci Technol, 46: 1940–1947
Mastalerz M, Gluskoter H, Rupp J. 2004. Carbon dioxide and methane sorption in high volatile bituminous coals from Indiana, USA. Int J Coal Geol, 60: 43–55
Mayo S L, Olafso B D, Goddard W A. 1990. Dreiding: A generic forcefield. J Phys Chem, 94: 8897–8909
Metropolis N, Rosenbluth A W, Marshall N, et al. 1953. Equation of state calculations by fast computing machines. J Chem Phys, 21: 1087–1092
Mosher K, He J J, Liu Y Y, et al. 2013. Molecular simulation of methane adsorption in micro- and mesoporous carbons with applications to coal and gas shale systems. Int J Coal Geol, 109–110: 36–44
Nakamura K, Takanohashi T, Lino M, et al. 1995. A model structure of Zao Zhuang bituminous coal. Energy Fuels, 9: 1003–1010
Narkiewicz M R, Mathews J P. 2009. Visual representation of carbon dioxide adsorption in a low-volatile bituminous coal molecular model. Energy Fuels, 23: 5236–5246
Nishino J. 2001. Adsorption of water vapor and carbon dioxide at carboxylic functional. Fuel, 80: 757–764
Ottiger S, Pini R, Storti G, et al. 2008. Competitive adsorption equilibria of CO2 and CH4 on a dry coal. Adsorption, 14: 539–556
Park S H, Sposito G. 2000. Monte Carlo simulation of total radial distribution functions for interlayer water in Li-, Na-, and K-Montmo-rillonite Hydrates. J Phys Chem B, 104: 4642–4648
Pini R, Ottiger S, Storti G, et al. 2009. Pure and competitive adsorption of CO2, CH4 and N2 on coal for ECBM. Energy Proc, 1: 1705–1710
Qin Y, Zhu W X. 2006. Several scientific problems faced in developing China’s coalbed methane industry (in Chinese). Bull Nat Natural Sci Foundation China, 20: 148–152
Romanov V. 2007. Coal chemistry for mechanical engineers: From macromolecular thermodynamics to reservoir simulation. Energy Fuels, 21: 1646–1654
Saghafi A, Faiz M, Roberts D. 2007. CO2 storage and gas diffusivity properties of coals from Sydney Basin, Australia. Int J Coal Geol, 70: 240–254
Shimada S, Li H Y, Oshima Y, et al. 2005. Displacement behavior of CH4 adsorbed on coals by injecting pure CO2, N2 and CO2-N2 mixture. Environ Geol. 49: 44–52
Su X B, Lin X Y. 2009. Coalbed Gas Geology (in Chinese). Beijing: China Coal Industry Publishing House. 119
Sun P D. 2001. Study on the mechanism of interaction for coal and methane gas (in Chinese). J Coal Sci Eng, 7: 58–63
Sun X Y, Li J W, Li Y X, et al. 2008. Adsorption of benzene and propene in β zeolite by grand canonical Monte Carlo simulation (in Chinese). Acta Chim Sin, 15: 1810–1814
Takanohashi T, Lino M, Nakamura K. 1998. Simulation of interaction of coal associates with solvents using the molecular dynamics calculation. Energy Fuels, 12: 1168–1173
Tambach T J, Mathews J P, Bergen F V. 2009. Molecular exchange of CH4 and CO2 in coal enhanced coalbed methane on a nanoscale. Energy Fuels, 23: 4845–4847
Tang S H, Tang D Z, Yang Q. 2004. Variation regularity of gas component concentration in binary-component gas adsorption-desorption isotherm experiments (in Chinese). J China Univ Mining Technol, 33: 448–452
Thomas G F. 1996. Coalbed methane potential of some Variscan foredeep basins. Geol Soc, 109: 13–26
Wang D Y, Xue C Y, Zhong C L. 2009. A molecular simulation of diffusion mechanism of n-alkanes in copper (II) benzene-1, 3, 5-tricarboxylate metal-organic framework (in Chinese). Acta Phys Sin, 8: 5552–5558
White C M, Smith D H, Jones K L, et al. 2005. Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery: A review. Energy Fuels, 19: 659–724
Xiang J H, Zeng F G, Liang H Z, et al. 2011. Model construction of the macromolecular structure of Yanzhou Coal and its molecular simulation. J Fuel Chem Technol, 39: 481–488
Yang K, Lu X C, Lin Y Z. 2010. Deformation of coal induced by methane adsorption at geological conditions. Energy Fuels, 24: 5955–5964
Yang Q Y, Zhong C L. 2006a. Electrostatic-field-induced enhancement of gas mixture separation in metal-organic frameworks: A computational study. ChemPhysChem, 7: 1417–1421
Yang Q Y, Zhong C L. 2006b. Molecular simulation of carbon dioxide/methane/hydrogen mixture adsorption in metal-organic frameworks. J Phys Chem B, 110: 17776–17783
Yu H G, Fan W T, Sun M Y, et al. 2005. Characteristics and predictions for adsorption isotherms of methane/carbon dioxide binary gas on coals (in Chinese). J China Coal Soc, 30: 618–622
Zhai G H, Duan L J, Tang S H, et al. 2012. Experimental study on CO2-coal interactions (in Chinese). J China Coal Soc, 37: 788–793
Zhang T J, Xu H J, Li S G, et al. 2009. The effect of temperature on the adsorbing capability of coal (in Chinese). J China Coal Soc, 34: 802–805
Zhang Z X, Liu G F, Zhang X D, et al. 2009. Adsorption-disorption experiments of CH4 and CO2 with different consistency (in Chinese). J China Coal Soc, 34: 551–555
Zheng Z. 2009. Molecular simulation study of the structure of shendong vitrinite and the adsorption of CH4, CO2 and H2O (in Chinese). Dissertation for Master Degree. Taiyuan: Taiyuan University of Technology
Zhong L W, Zheng Y Z, Yun Z R, et al. 2002. The adsorption capability of coal under integrated influence of temperature and pressure and predicted of content quantity of coal bed gas (in Chinese). J China Coal Soc, 27: 581–585
Zhou L, Feng Q Y, Qin Y. 2011. Thermodynamic analysis of competitive adsorption of CO2 and CH4 on coal matrix (in Chinese). J China Coal Soc, 36: 1307–1311
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiang, J., Zeng, F., Liang, H. et al. Molecular simulation of the CH4/CO2/H2O adsorption onto the molecular structure of coal. Sci. China Earth Sci. 57, 1749–1759 (2014). https://doi.org/10.1007/s11430-014-4849-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11430-014-4849-9