Abstract
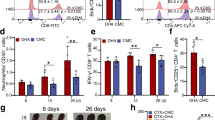
Artemisia annua is an anti-fever herbal medicine first described in traditional Chinese medicine 1,000 years ago. Artemisinin, the extract of A. annua, and its derivatives (dihydroartemisinin (DHA), artemether, and artesunate) have been used for the treatment of malaria with substantial efficacy. Recently, DHA has also been tested for the treatment of lupus erythematosus, indicating that it may function to balance the immune response in immunocompromised individuals. In the present study, the regulatory effect of artemisinin on the murine immune system was systematically investigated in mice infected with two different protozoan parasites (Toxoplasma gondii and Plasmodium berghei). Our results revealed that the mouse spleen index significantly increased (spleen enlargement) in the healthy mice after DHA administration primarily due to the generation of an extra number of lymphocytes and CD8+ T lymphocytes in both the spleen and circulation. DHA could increase the proportion of T helper cells and CD8+ T cells, as well as decrease the number of splenic and circulatory B cells. Further, DHA could reduce the production of proinflammatory cytokines. Our study revealed that apart from their anti-parasitic activity, artemisinin and its derivatives can also actively modulate the immune system to directly benefit the host.
Similar content being viewed by others
References
Abdin, A.A., Ashour, D.S., and Shoheib, Z.S. (2013). Artesunate effect on schistosome thioredoxin glutathione reductase and cytochrome c peroxidase as new molecular targets in Schistosoma mansoni-infected mice. Biomed Environ Sci, 26, 953–961.
Aderka, D., Wysenbeek, A., Engelmann, H., Cope, A.P., Brennan, F., Molad, Y., Hornik, V., Levo, Y., Maini, R.N., Feldmann, M., et al. (2010). Correlation between serum levels of soluble tumor necrosis factor receptor and disease activity in systemic lupus erythematosus. Arthrit Rheumat 36, 1111–1120.
Bhadra, R., Gigley, J.P., and Khan, I.A. (2011a). The CD8 T-cell road to immunotherapy of toxoplasmosis. Immunotherapy 3, 789–801.
Bhadra, R., Gigley, J.P., Weiss, L.M., and Khan, I.A. (2011b). Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci USA 108, 9196–9201.
Cheng, C., Ho, W.E., Goh, F.Y., Guan, S.P., Kong, L.R., Lai, W.Q., Leung, B.P., and Wong, W.S.F. (2011). Anti-malarial drug artesunate attenuates experimental allergic asthma via inhibition of the phosphoinositide 3-kinase/Akt pathway. PLoS ONE 6, e20932.
Chimanuka, B., Francois, G., Timperman, G., Heyden, Y.V., Holenz, J., Plaizier-Vercammen, J., and Bringmann, G. (2001). A comparison of the stage-specific efficacy of chloroquine, artemether and dioncophylline B against the rodent malaria parasite Plasmodium chabaudi chabaudi in vivo. Parasitol Res 87, 795–803.
Couper, K.N., Roberts, C.W., Brombacher, F., Alexander, J., and Johnson, L.L. (2005). Toxoplasma gondii-specific immunoglobulin M limits parasite dissemination by preventing host cell invasion. Infect Immun 73, 8060–8068.
Cruz-González, D.J., Gómez-Martin, D., Layseca-Espinosa, E., Baranda, L., Abud-Mendoza, C., Alcocer-Varela, J., González-Amaro, R., and Monsiváis-Urenda, A.E. (2018). Analysis of the regulatory function of natural killer cells from patients with systemic lupus erythematosus. Clin Exp Immunol 191, 288–300.
Dong, Y.J., Li, W.D., and Tu, Y.Y. (2003) Effect of dihydro-qinghaosu on auto-antibody production, TNF alpha secretion and pathologic change of lupus nephritis in BXSB mice (in Chinese). Zhongguo Zhong Xi Yi Jie He Za Zhi 23, 676–679.
Du, X.X., Li, Y.J., Wu, C.L., Zhou, J.H., Han, Y., Sui, H., Wei, X.L., Liu, L., Huang, P., Yuan, H.H., et al. (2013). Initiation of apoptosis, cell cycle arrest and autophagy of esophageal cancer cells by dihydroartemisinin. Biomed Pharmacother 67, 417–424.
Dunay, I.R., Chan, W.C., Haynes, R.K., and Sibley, L.D. (2009). Artemisone and artemiside control acute and reactivated toxoplasmosis in a murine model. Antimicrobial Agents Chemother 53, 4450–4456.
Efferth, T. (2017). From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Seminars Cancer Biol 46, 65–83.
Feng, Y., Zhu, X., Wang, Q., Jiang, Y., Shang, H., Cui, L., and Cao, Y. (2012). Allicin enhances host pro-inflammatory immune responses and protects against acute murine malaria infection. Malar J 11, 268.
Gordon, C., Li, C.K., and Isenberg, D.A. (2009). Systemic lupus erythematosus. N Engl J Med 38, 73–80.
Guo, Y., Xu, P., Xuan, Y., Wu, L., and Li, S. (1997) Effect of artesunate on vltrastructure of schistosomula Schistosoma japonicum (in Chinese). Chin J Schistosom Contr 9, 34–36.
He, Y., Fan, J., Lin, H., Yang, X., Ye, Y., Liang, L., Zhan, Z., Dong, X., Sun, L., and Xu, H. (2011). The anti-malaria agent artesunate inhibits expression of vascular endothelial growth factor and hypoxia-inducible factor-1α in human rheumatoid arthritis fibroblast-like synoviocyte. Rheumatol Int 31, 53–60.
Hou, L.F., He, S.J., Li, X., Yang, Y., He, P.L., Zhou, Y., Zhu, F.H., Yang, Y. F., Li, Y., Tang, W., et al. (2011). Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthrit Rheumat 63, 2445–2455.
Hou, L., Block, K.E., and Huang, H. (2014). Artesunate abolishes germinal center B cells and inhibits autoimmune arthritis. PLoS ONE 9, e104762.
Huang, X., Xie, Z., Liu, F., Han, C., Zhang, D., Wang, D., Bao, X., Sun, J., Wen, C., and Fan, Y. (2014). Dihydroartemisinin inhibits activation of the Toll-like receptor 4 signaling pathway and production of type I interferon in spleen cells from lupus-prone MRL/lpr mice. Int Immunopharmacol 22, 266–272.
Klonis, N., Crespo-Ortiz, M.P., Bottova, I., Abu-Bakar, N., Kenny, S., Rosenthal, P.J., and Tilley, L. (2011). Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc Natl Acad Sci USA 108, 11405–11410.
Langermans, J.A., Van der Hulst, M.E., Nibbering, P.H., Hiemstra, P.S., Fransen, L., and Van Furth, R. (1992). IFN-gamma-induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol 148, 568–574.
Lewis, J.E., Fu, S.M., and Gaskin, F. (2013). Autoimmunity, end organ damage, and the origin of autoantibodies and autoreactive T cells in systemic lupus erythematosus. Discov Med 15, 85–92.
Li, G.Q., Guo, X.B., Fu, L.C., Jian, H.X., and Wang, X.H. (1994). Clinical trials of artemisinin and its derivatives in the treatment of malaria in China. Trans R Soc Trop Med Hygiene 88, 5–6.
Li, T., Chen, H., Wei, N., Mei, X., Zhang, S., Liu, D., Gao, Y., Bai, S., Liu, X., and Zhou, Y. (2012). Anti-inflammatory and immunomodulatory mechanisms of artemisinin on contact hypersensitivity. Int Immunopharmacol 12, 144–150.
Li, T., Chen, H., Yang, Z., Liu, X.G., Zhang, L.M., and Wang, H. (2013a). Evaluation of the immunosuppressive activity of artesunate in vitro and in vivo. Int Immunopharmacol 16, 306–312.
Li, W., Dong, Y., Tu, Y., and Lin, Z. (2006). Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signaling pathway NF-kappa B translocation. Int Immunopharmacol 6, 1243–1250.
Li, X., Li, T.T., Zhang, X.H., Hou, L.F., Yang, X.Q., Zhu, F.H., Tang, W., and Zuo, J.P. (2013b). Artemisinin analogue SM934 ameliorates murine experimental autoimmune encephalomyelitis through enhancing the expansion and functions of regulatory T cell. PLoS ONE 8, e74108.
Li, Y. (2012). Qinghaosu (artemisinin): Chemistry and pharmacology. Acta Pharmacol Sin 33, 1141–1146.
Listed, N. (1979). Antimalaria studies on Qinghaosu. Chin Med J (Engl) 92, 811–816.
Lourenco, E.V., Procaccini, C., Ferrera, F., Iikuni, N., Singh, R.P., Filaci, G., Matarese, G., Shi, F.D., Brahn, E., Hahn, B.H., et al. (2009). Modulation of p38 MAPK activity in regulatory T cells after tolerance with anti-DNA Ig peptide in (NZB × NZW)F1 lupus mice. J Immunol 182, 7415–7421.
Mack, D.G., and Mcleod, R. (1992). Human Toxoplasma gondii-specific secretory immunoglobulin A reduces T. gondii infection of enterocytes in vitro. J Clin Invest 90, 2585–2592.
Mahmoudvand, H., Ziaali, N., Ghazvini, H., Shojaee, S., Keshavarz, H., Esmaeilpour, K., and Sheibani, V. (2016). Toxoplasma gondii infection promotes neuroinflammation through cytokine networks and induced hyperalgesia in BALB/c mice. Inflammation 39, 405–412.
Matowicka-Karna, J., Dymicka-Piekarska, V., and Kemona, H. (2009). Does Toxoplasma gondii Infection Affect the Levels of IgE and Cytokines (IL-5, IL-6, IL-10, IL-12, and TNF-alpha)? Clin Dev Immunol 2009(1), 1–4.
Meira, C.S., Pereira-Chioccola, V.L., Vidal, J.E., de Mattos, C.C.B., Motoie, G., Costa-Silva, T.A., Gava, R., Frederico, F.B., and de Mattos, L.C. (2014). Cerebral and ocular toxoplasmosis related with IFN-γ, TNF-α, and IL-10 levels. Front Microbiol 5, 492.
Meshnick, S.R. (2002). Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol 32, 1655–1660.
Munoz, M., Liesenfeld, O., and Heimesaat, M.M. (2015). Immunology of Toxoplasma gondii.Immunol Rev 240, 269–285.
Nagamune, K., Beatty, W.L., and Sibley, L.D. (2007). Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot Cell 6, 2147–2156.
Qinghaosu Research Group, Institute of Biophysics Academia Sinica. (1980). Crystal structure and absolute configuration of Qinghaosu. Sci China Ser A, 380–396.
Shakoor, N., Michalska, M., Harris, C.A., and Block, J.A. (2002). Drug-induced systemic lupus erythematosus associated with etanercept therapy. Lancet 359, 579–580.
Schofield, L., Villaquiran, J., Ferreira, A., Schellekens, H., Nussenzweig, R., and Nussenzweig, V. (1987). γ Interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330, 664–666.
Shi, X., Wang, L., Li, X., Bai, J., Li, J., Li, S., Wang, Z., and Zhou, M. (2017). Dihydroartemisinin induces autophagy-dependent death in human tongue squamous cell carcinoma cells through DNA doublestrand break-mediated oxidative stress. Oncotarget 8, 45981–45993.
Shlomchik, M.J., Craft, J.E., and Mamula, M.J. (2001). From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol 1, 147–153.
Sibley, L.D., Adams, L.B., Fukutomi, Y., and Krahenbuhl, J.L. (1991). Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol 147, 2340–2345.
Skinner, T.S., Manning, L.S., Johnston, W.A., and Davis, T.M.E. (1996). In vitro stage-specific sensitivity of Plasmodium falciparum to quinine and artemisinin drugs. Int J Parasitol 26, 519–525.
Studnicka-Benke, A., Steiner, G., Petera, P., and Smolen, J.S. (1996). Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Rheumatology 35, 1067–1074.
Stumhofer, J.S., Laurence, A., Wilson, E.H., Huang, E., Tato, C.M., Johnson, L.M., Villarino, A.V., Huang, Q., Yoshimura, A., Sehy, D., et al. (2006). Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 7, 937–945.
Sun, H., Meng, X., Han, J., Zhang, Z., Wang, B., Bai, X., and Zhang, X. (2013). Anti-cancer activity of DHA on gastric cancer—an in vitro and in vivo study. Tumor Biol 34, 3791–3800.
Tajima, M., Wakita, D., Noguchi, D., Chamoto, K., Yue, Z., Fugo, K., Ishigame, H., Iwakura, Y., Kitamura, H., and Nishimura, T. (2008). IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med 205, 1019–1027.
Tsokos, G.C. (2011). Systemic lupus erythematosus. N Engl J Med 365, 2110–2121.
Utzinger, J., Chollet, J., You, J., Mei, J., Tanner, M., and Xiao, S. (2001). Effect of combined treatment with praziquantel and artemether on Schistosoma japonicum and Schistosoma mansoni in experimentally infected animals. Acta Trop 80, 9–18.
Villegas-Mendez, A., de Souza, J.B., Murungi, L., Hafalla, J.C.R., Shaw, T. N., Greig, R., Riley, E.M., and Couper, K.N. (2011). Heterogeneous and tissue-specific regulation of effector T cell responses by IFN-gamma during Plasmodium berghei ANKA infection. J Immunol 187, 2885–2897.
Wilson, M.S., Feng, C.G., Barber, D.L., Yarovinsky, F., Cheever, A.W., Sher, A., Grigg, M., Collins, M., Fouser, L., and Wynn, T.A. (2010). Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol 184, 4378–4390.
Wu, L., Xu, Y., Guo, Y., Xu, P., and Li, S. (1996). Studies on the effect of artesunate to the energy-metabolic enzymes (in Chinese). Chin J Schistosom Contr 5, 267–269.
Wen, X., Zhang, D., Kikuchi, Y., Jiang, Y., Nakamura, K., Xiu, Y., Tsurui, H., Takahashi, K., Abe, M., Ohtsuji, M., et al. (2004). Transgene-mediated hyper-expression of IL-5 inhibits autoimmune disease but increases the risk of B cell chronic lymphocytic leukemia in a model of murine lupus. Eur J Immunol 34, 2740–2749.
Xiao, S.H., Booth, M., and Tanner, M. (2000). The prophylactic effects of artemether against Schistosoma japonicum infections. Parasitol Today 16, 122–126.
Xing, C., Zhu, G., Xiao, H., Fang, Y., Liu, X., Han, G., Chen, G., Hou, C., Shen, B., Li, Y., et al. (2017). B cells regulate thymic CD8+T cell differentiation in lupus-prone mice. Oncotarget 8, 89486–89499.
Xu, C.H., Liu, Y., Xiao, L.M., Guo, C.G., Zheng, S.Y., Zeng, E.M., and Li, D.H. (2017). Dihydroartemisinin treatment exhibits antitumor effects in glioma cells through induction of apoptosis. Mol Med Rep 16, 9528–9532.
Xu, L.M., Chen, X.R., and Tu, Y.Y. (2002). Effect of hydroartemisinin on lupus BXSB mice (in Chinese). Chin J Dermatovenerol Integr Trad West Med 1, 19–20.
Xu, H., He, Y., Yang, X., Liang, L., Zhan, Z., Ye, Y., Yang, X., Lian, F., and Sun, L. (2007). Anti-malarial agent artesunate inhibits TNF-alpha-induced production of proinflammatory cytokines via inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology 46, 920–926.
Zhang, S., Shi, L., Ma, H., Li, H., Li, Y., Lu, Y., Wang, Q., and Li, W. (2017). Dihydroartemisinin induces apoptosis in human gastric cancer cell line BGC-823 through activation of JNK1/2 and p38 MAPK signaling pathways. J Recept Signal Transduct 37, 174–180.
Zhao, X., Zhong, H., Wang, R., Liu, D., Waxman, S., Zhao, L., and Jing, Y. (2015) Dihydroartemisinin and its derivative induce apoptosis in acute myeloid leukemia through Noxa-mediated pathway requiring iron and endoperoxide moiety. Oncotarget 6, 5582–5596.
Zhao, Y.G., Wang, Y., Guo, Z., Gu, A., Dan, H.C., Baldwin, A.S., Hao, W., and Wan, Y.Y. (2012). Dihydroartemisinin ameliorates inflammatory disease by its reciprocal effects on Th and regulatory T cell function via modulating the mammalian target of rapamycin pathway. J Immunol 189, 4417–4425.
Zhou, W., Wu, J., Wu, Q., Wang, J., Zhou, Y., Zhou, R., He, P., Li, X., Yang, Y., Zhang, Y., et al. (2010). A novel artemisinin derivative, 3-(12-beta-artemisininoxy) phenoxyl succinic acid (SM735), mediates immunosuppressive effects in vitro and in vivo. Acta Pharmacol Sin 26, 1352–1358.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFD0500400), the National Natural Science Foundation of China (81420108023, 81772219) and distinguished scientist grant from Shenyang Agricultural University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance and ethics The author(s) declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, T., Zhang, Y., Jiang, N. et al. Dihydroartemisinin regulates the immune system by promotion of CD8+ T lymphocytes and suppression of B cell responses. Sci. China Life Sci. 63, 737–749 (2020). https://doi.org/10.1007/s11427-019-9550-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-019-9550-4