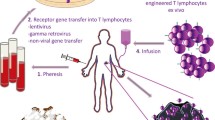
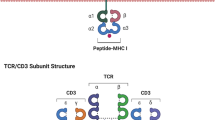
Abstract
The host immune system plays an instrumental role in the surveillance and elimination of tumors by recognizing and destroying cancer cells. In recent decades, studies have mainly focused on adoptive immunotherapy using engineered T cells for the treatment of malignant diseases. Through gene engraftment of the patient’s own T cells with chimeric antigen receptor (CAR), they can recognize tumor specific antigens effectively and eradicate selectively targeted cells in an MHC-independent fashion. To date, CAR-T cell therapy has shown great clinical utility in patients with B-cell leukemias. Owing to different CAR designs and tumor complex microenvironments, genetically redirected T cells may generate diverse biological properties and thereby impact their long-term clinical performance and outcome. Meanwhile some unexpected toxicities that result from CAR-T cell application have been examined and limited the curative effects. Diverse important parameters are closely related with adoptively transferred cell behaviors, including CAR-T cells homing, CAR constitutive signaling, T cell differentiation and exhaustion. Thus, understanding CARs molecular design to improve infused cell efficacy and safety is crucial to clinicians and patients who are considering this novel cancer therapeutics. In this review, the developments in CAR-T cell therapy and the limitations and perspectives in optimizing this technology towards clinical application are discussed.
Similar content being viewed by others
References
Adachi, K., Kano, Y., Nagai, T., Okuyama, N., Sakoda, Y., and Tamada, K. (2018a). IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol 36, 346–351.
Adachi, K., Kano, Y., Nagai, T., Okuyama, N., Sakoda, Y., and Tamada, K. (2018b). IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol 36, 346–351.
Appleman, L.J., van Puijenbroek, A.A.F.L., Shu, K.M., Nadler, L.M., and Boussiotis, V.A. (2002). CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol 168, 2729–2736.
Arcangeli, S., Rotiroti, M.C., Bardelli, M., Simonelli, L., Magnani, C.F., Biondi, A., Biagi, E., Tettamanti, S., and Varani, L. (2017). Balance of Anti-CD123 Chimeric antigen receptor binding affinity and density for the targeting of acute myeloid leukemia. Mol Ther 25, 1933–1945.
Bak, S.P., Barnkob, M.S., Bai, A., Higham, E.M., Wittrup, K.D., and Chen, J. (2012). Differential requirement for CD70 and CD80/CD86 in dendritic cell-mediated activation of tumor-tolerized CD8 T cells. J Immunol 189, 1708–1716.
Baruch, E.N., Berg, A.L., Besser, M.J., Schachter, J., and Markel, G. (2017). Adoptive T cell therapy: an overview of obstacles and opportunities. Cancer 123, 2154–2162.
Beavis, P.A., Henderson, M.A., Giuffrida, L., Mills, J.K., Sek, K., Cross, R. S., Davenport, A.J., John, L.B., Mardiana, S., Slaney, C.Y., et al. (2017). Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clinical Investigation 127, 929–941.
Bonifant, C.L., Jackson, H.J., Brentjens, R.J., and Curran, K.J. (2016). Toxicity and management in CAR T-cell therapy. Mol Ther - Oncolytics 3, 16011.
Brentjens, R.J., Davila, M.L., Riviere, I., Park, J., Wang, X., Cowell, L.G., Bartido, S., Stefanski, J., Taylor, C., Olszewska, M., et al. (2013). CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Translational Med 5, 177ra38.
Bridgeman, J.S., Hawkins, R.E., Hombach, A.A., Abken, H., and Gilham, D.E. (2010). Building better chimeric antigen receptors for adoptive T cell therapy. CGT 10, 77–90.
Brown, C.E., Alizadeh, D., Starr, R., Weng, L., Wagner, J.R., Naranjo, A., Ostberg, J.R., Blanchard, M.S., Kilpatrick, J., Simpson, J., et al. (2016). Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 375, 2561–2569.
Cao, Y., Rodgers, D.T., Du, J., Ahmad, I., Hampton, E.N., Ma, J.S.Y., Mazagova, M., Choi, S.H., Yun, H.Y., Xiao, H., et al. (2016). Design of switchable chimeric antigen receptor T cells targeting breast cancer. Angew Chem Int Ed 55, 7520–7524.
Cartellieri, M., Feldmann, A., Koristka, S., Arndt, C., Loff, S., Ehninger, A., von Bonin, M., Bejestani, E.P., Ehninger, G., and Bachmann, M.P. (2016). Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J 6, e458.
Cheadle, E.J., Rothwell, D.G., Bridgeman, J.S., Sheard, V.E., Hawkins, R. E., and Gilham, D.E. (2012). Ligation of the CD2 co-stimulatory receptor enhances IL-2 production from first-generation chimeric antigen receptor T cells. Gene Ther 19, 1114–1120.
Chen, C., Li, K., Jiang, H., Song, F., Gao, H., Pan, X., Shi, B., Bi, Y., Wang, H., Wang, H., et al. (2017). Development of T cells carrying two complementary chimeric antigen receptors against glypican-3 and asialoglycoprotein receptor 1 for the treatment of hepatocellular carcinoma. Cancer Immunol Immunother 66, 475–489.
Choi, B.D., Suryadevara, C.M., Gedeon, P.C., Herndon II, J.E., Sanchez- Perez, L., Bigner, D.D., and Sampson, J.H. (2014). Intracerebral delivery of a third generation EGFRvIII-specific chimeric antigen receptor is efficacious against human glioma. J Clinical Neuroscience 21, 189–190.
Dai, H., Wang, Y., Lu, X., and Han, W. (2016). Chimeric antigen receptors modified T-cells for cancer therapy. J Natl Cancer Inst 108.
Davenport, A.J., Cross, R.S., Watson, K.A., Liao, Y., Shi, W., Prince, H.M., Beavis, P.A., Trapani, J.A., Kershaw, M.H., Ritchie, D.S., et al. (2018). Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc Natl Acad Sci USA 115, E2068–E2076.
Diaconu, I., Ballard, B., Zhang, M., Chen, Y., West, J., Dotti, G., and Savoldo, B. (2017). Inducible caspase-9 selectively modulates the toxicities of CD19-specific chimeric antigen receptor-modified T Cells. Mol Ther 25, 580–592.
Ding, G., and Chen, H. (2016). Adoptive transfer of T cells transduced with a chimeric antigen receptor to treat relapsed or refractory acute leukemia: efficacy and feasibility of immunotherapy approaches. Sci China Life Sci 59, 673–677.
Dong, G., Kalifa, R., Nath, P.R., Gelkop, S., and Isakov, N. (2017). TCR crosslinking promotes Crk adaptor protein binding to tyrosinephosphorylated CD3? chain. Biochem BioPhys Res Commun 488, 541–546.
Feldmann, A., Arndt, C., Bergmann, R., Loff, S., Cartellieri, M., Bachmann, D., Aliperta, R., Hetzenecker, M., Ludwig, F., Albert, S., et al. (2017). Retargeting of T lymphocytes to PSCA- or PSMA positive prostate cancer cells using the novel modular chimeric antigen receptor platform technology “UniCAR”. Oncotarget 8, 31368–31385.
Feng, K.C., Guo, Y.L., Liu, Y., Dai, H.R., Wang, Y., Lv, H.Y., Huang, J.H., Yang, Q.M., and Han, W.D. (2017a). Cocktail treatment with EGFRspecific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol 10, 4.
Feng, M., Xiong, G., Cao, Z., Yang, G., Zheng, S., Song, X., You, L., Zheng, L., Zhang, T., and Zhao, Y. (2017b). PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett 407, 57–65.
Fesnak, A.D., June, C.H., and Levine, B.L. (2016). Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 16, 566–581.
Finney, H.M., Lawson, A.D., Bebbington, C.R., and Weir, A.N. (1998). Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol 161, 2791–2797.
Fry, T.J., Shah, N.N., Orentas, R.J., Stetler-Stevenson, M., Yuan, C.M., Ramakrishna, S., Wolters, P., Martin, S., Delbrook, C., Yates, B., et al. (2018). CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 24, 20–28.
Gao, H., Li, K., Tu, H., Pan, X., Jiang, H., Shi, B., Kong, J., Wang, H., Yang, S., Gu, J., et al. (2014). Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clinical Cancer Res 20, 6418–6428.
Gardner, R.A., Finney, O., Annesley, C., Brakke, H., Summers, C., Leger, K., Bleakley, M., Brown, C., Mgebroff, S., Kelly-Spratt, K.S., et al. (2017). Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129, 3322–3331.
Golumba-Nagy, V., Kuehle, J., Hombach, A.A., and Abken, H. (2018). CD28-? CAR T cells resist TGF-ß repression through IL-2 signaling, which can be mimicked by an engineered IL-7 autocrine loop. Mol Ther 26, 2218–2230.
Grinberg-Bleyer, Y., Oh, H., Desrichard, A., Bhatt, D.M., Caron, R., Chan, T.A., Schmid, R.M., Klein, U., Hayden, M.S., and Ghosh, S. (2017). NF-kB c-Rel is crucial for the regulatory T cell immune checkpoint in cancer. Cell 170, 1096–1108.e13.
Gross, G., Waks, T., and Eshhar, Z. (1989). Expression of immunoglobulin- T-cell receptor chimeric molecules as functional receptors with antibody-type specificity.. Proc Natl Acad Sci USA 86, 10024–10028.
Guo, Y., Feng, K., Liu, Y., Wu, Z., Dai, H., Yang, Q., Wang, Y., Jia, H., and Han, W. (2018). Phase I study of chimeric antigen receptor-modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin Cancer Res 24, 1277–1286.
Han, S., Latchoumanin, O., Wu, G., Zhou, G., Hebbard, L., George, J., and Qiao, L. (2017). Recent clinical trials utilizing chimeric antigen receptor T cells therapies against solid tumors. Cancer Lett 390, 188–200.
Harton, J., Jin, L., Hahn, A., and Drake, J. (2016). Immunological functions of the membrane proximal region of MHC class II molecules. F1000Res 5.
Holohan, D.R., Lee, J.C., and Bluestone, J.A. (2015). Shifting the evolving CAR T cell platform into higher gear. Cancer Cell 28, 401–402.
Hombach, A., Hombach, A.A., and Abken, H. (2010). Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther 17, 1206–1213.
Hombach, A.A., and Abken, H. (2011). Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int J Cancer 129, 2935–2944.
Hu, Y., Wu, Z., Luo, Y., Shi, J., Yu, J., Pu, C., Liang, Z., Wei, G., Cui, Q., Sun, J., et al. (2017). Potent anti-leukemia activities of chimeric antigen receptor-modified T cells against CD19 in Chinese patients with relapsed/refractory acute lymphocytic leukemia. Clin Cancer Res 23, 3297–3306.
Hudecek, M., Sommermeyer, D., Kosasih, P.L., Silva-Benedict, A., Liu, L., Rader, C., Jensen, M.C., and Riddell, S.R. (2015). The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res 3, 125–135.
Hwang, W.T., Adams, S.F., Tahirovic, E., Hagemann, I.S., and Coukos, G. (2012). Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecologic Oncology 124, 192–198.
Juillerat, A., Marechal, A., Filhol, J.M., Valogne, Y., Valton, J., Duclert, A., Duchateau, P., and Poirot, L. (2017). An oxygen sensitive self-decision making engineered CAR T-cell. Sci Rep 7, 39833.
Kershaw, M.H., Westwood, J.A., Parker, L.L., Wang, G., Eshhar, Z., Mavroukakis, S.A., White, D.E., Wunderlich, J.R., Canevari, S., Rogers-Freezer, L., et al. (2006). A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clinical Cancer Res 12, 6106–6115.
Klein-Hessling, S., Muhammad, K., Klein, M., Pusch, T., Rudolf, R., Flöter, J., Qureischi, M., Beilhack, A., Vaeth, M., Kummerow, C., et al. (2017). NFATc1 controls the cytotoxicity of CD8+ T cells. Nat Commun 8, 511.
Kong, S., Sengupta, S., Tyler, B., Bais, A.J., Ma, Q., Doucette, S., Zhou, J., Sahin, A., Carter, B.S., Brem, H., et al. (2012). Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clinical Cancer Res 18, 5949–5960.
Krenciute, G., Krebs, S., Torres, D., Wu, M.F., Liu, H., Dotti, G., Li, X.N., Lesniak, M.S., Balyasnikova, I.V., and Gottschalk, S. (2016). Characterization and functional analysis of scFv-based chimeric antigen receptors to redirect T cells to IL13Ra2-positive glioma. Mol Ther 24, 354–363.
Krug, C., Birkholz, K., Paulus, A., Schwenkert, M., Schmidt, P., Hoffmann, N., Hombach, A., Fey, G., Abken, H., Schuler, G., et al. (2015). Stability and activity of MCSP-specific chimeric antigen receptors (CARs) depend on the scFv antigen-binding domain and the protein backbone. Cancer Immunol Immunother 64, 1623–1635.
Lai, Y., Weng, J., Wei, X., Qin, L., Lai, P., Zhao, R., Jiang, Z., Li, B., Lin, S., Wang, S., et al. (2018). Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T Cells. Leukemia 32, 801–808.
Lamers, C.H.J., Klaver, Y., Gratama, J.W., Sleijfer, S., and Debets, R. (2016). Treatment of metastatic renal cell carcinoma (mRCC) with CAIX CAR-engineered T-cells-a completed study overview. Biochem Soc Trans 44, 951–959.
Lamers, C.H., Sleijfer, S., van Steenbergen, S., van Elzakker, P., van Krimpen, B., Groot, C., Vulto, A., den Bakker, M., Oosterwijk, E., Debets, R., et al. (2013). Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 21, 904–912.
Lanitis, E., Poussin, M., Klattenhoff, A.W., Song, D., Sandaltzopoulos, R., June, C.H., and Powell, D.J. (2013). Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res 1, 43–53.
Li, D., and Wang, W. (2017). Booming cancer immunotherapy fighting tumors. Sci China Life Sci 60, 1445–1449.
Li, H., and Zhao, Y. (2017). Increasing the safety and efficacy of chimeric antigen receptor T cell therapy. Protein Cell 8, 573–589.
Li, N., Liu, S., Sun, M., Chen, W., Xu, X., Zeng, Z., Tang, Y., Dong, Y., Chang, A.H., and Zhao, Q. (2018). Chimeric antigen receptor-modified T cells redirected to EphA2 for the immunotherapy of non-small cell lung cancer. Translational Oncology 11, 11–17.
Li, S., Yang, Z., Shen, J., Shan, J., and Qian, C. (2016). Adoptive therapy with CAR redirected T cells for hematological malignancies. Sci China Life Sci 59, 370–378.
Ligtenberg, M.A., Mougiakakos, D., Mukhopadhyay, M., Witt, K., Lladser, A., Chmielewski, M., Riet, T., Abken, H., and Kiessling, R. (2016). Coexpressed catalase protects chimeric antigen receptor-redirected T cells as well as bystander cells from oxidative stress-induced loss of antitumor activity. JI 196, 759–766.
Lo, A.S.Y., Xu, C., Murakami, A., and Marasco, W.A. (2014). Regression of established renal cell carcinoma in nude mice using lentivirustransduced human T cells expressing a human anti-CAIX chimeric antigen receptor. Mol Ther - Oncolytics 1, 14003.
Louis, C.U., Savoldo, B., Dotti, G., Pule, M., Yvon, E., Myers, G.D., Rossig, C., Russell, H.V., Diouf, O., Liu, E., et al. (2011). Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056.
Luo, C., Wei, J., and Han, W. (2016). Spotlight on chimeric antigen receptor engineered T cell research and clinical trials in China. Sci China Life Sci 59, 349–359.
Maciocia, P.M., Wawrzyniecka, P.A., Philip, B., Ricciardelli, I., Akarca, A. U., Onuoha, S.C., Legut, M., Cole, D.K., Sewell, A.K., Gritti, G., et al. (2017). Targeting the T cell receptor ß-chain constant region for immunotherapy of T cell malignancies. Nat Med 23, 1416–1423.
Mata, M., Gerken, C., Nguyen, P., Krenciute, G., Spencer, D.M., and Gottschalk, S. (2017). Inducible activation of MyD88 and CD40 in CAR T-cells results in controllable and potent antitumor activity in preclinical solid tumor models. Cancer Discov.
Maude, S.L., Frey, N., Shaw, P.A., Aplenc, R., Barrett, D.M., Bunin, N.J., Chew, A., Gonzalez, V.E., Zheng, Z., Lacey, S.F., et al. (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371, 1507–1517.
Mbofung, R.M., McKenzie, J.A., Malu, S., Zhang, M., Peng, W., Liu, C., Kuiatse, I., Tieu, T., Williams, L., Devi, S., et al. (2017). HSP90 inhibition enhances cancer immunotherapy by upregulating interferon response genes. Nat Commun 8, 451.
Milone, M.C., Fish, J.D., Carpenito, C., Carroll, R.G., Binder, G.K., Teachey, D., Samanta, M., Lakhal, M., Gloss, B., Danet-Desnoyers, G., et al. (2009). Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 17, 1453–1464.
Morello, A., Sadelain, M., and Adusumilli, P.S. (2016). Mesothelintargeted CARs: driving T cells to solid tumors. Cancer Discovery 6, 133–146.
Morgan, R.A., Yang, J.C., Kitano, M., Dudley, M.E., Laurencot, C.M., and Rosenberg, S.A. (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18, 843–851.
Moritz, D., Wels, W., Mattern, J., and Groner, B. (1994). Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2- expressing tumor cells.. Proc Natl Acad Sci USA 91, 4318–4322.
Neelapu, S.S., Tummala, S., Kebriaei, P., Wierda, W., Locke, F.L., Lin, Y., Jain, N., Daver, N., Gulbis, A.M., Adkins, S., et al. (2018). Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘ALL’. Nat Rev Clin Oncol 15, 218.
O’Rourke, D.M., Nasrallah, M.P., Desai, A., Melenhorst, J.J., Mansfield, K., Morrissette, J.J.D., Martinez-Lage, M., Brem, S., Maloney, E., Shen, A., et al. (2017). A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 9, pii: eaaa0984.
Oberschmidt, O., Kloess, S., and Koehl, U. (2017). Redirected primary human chimeric antigen receptor natural killer cells as an “off-the-shelf immunotherapy” for improvement in cancer treatment. Front Immunol 8, 654.
Pellegatta, S., Savoldo, B., Di Ianni, N., Corbetta, C., Chen, Y., Patané, M., Sun, C., Pollo, B., Ferrone, S., DiMeco, F., et al. (2018). Constitutive and TNFa-inducible expression of chondroitin sulfate proteoglycan 4 in glioblastoma and neurospheres: Implications for CAR-T cell therapy. Sci Transl Med 10, eaao2731.
Petersen, C.T., Hassan, M., Morris, A.B., Jeffery, J., Lee, K., Jagirdar, N., Staton, A.D., Raikar, S.S., Spencer, H.T., Sulchek, T., et al. (2018). Improving T-cell expansion and function for adoptive T-cell therapy using ex vivo treatment with PI3Kd inhibitors and VIP antagonists. Blood Adv 2, 210–223.
Pishali Bejestani, E., Cartellieri, M., Bergmann, R., Ehninger, A., Loff, S., Kramer, M., Spehr, J., Dietrich, A., Feldmann, A., Albert, S., et al. (2017). Characterization of a switchable chimeric antigen receptor platform in a pre-clinical solid tumor model. OncoImmunology 6, e1342909.
Porter, D.L., Levine, B.L., Kalos, M., Bagg, A., and June, C.H. (2011). Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365, 725–733.
Roybal, K.T., Rupp, L.J., Morsut, L., Walker, W.J., McNally, K.A., Park, J. S., and Lim, W.A. (2016). Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 164, 770–779.
Ruella, M., Barrett, D.M., Kenderian, S.S., Shestova, O., Hofmann, T.J., Perazzelli, J., Klichinsky, M., Aikawa, V., Nazimuddin, F., Kozlowski, M., et al. (2016). Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clinical Investigation 126, 3814–3826.
Sadelain, M. (2015). CAR therapy: the CD19 paradigm. J Clinical Investigation 125, 3392–3400.
Sadelain, M., Brentjens, R., and Rivière, I. (2013). The basic principles of chimeric antigen receptor design. Cancer Discovery 3, 388–398.
Sanmamed, M.F., Pastor, F., Rodriguez, A., Perez-Gracia, J.L., Rodriguez- Ruiz, M.E., Jure-Kunkel, M., and Melero, I. (2015). Agonists of costimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Seminars Oncology 42, 640–655.
Santoro, S.P., Kim, S., Motz, G.T., Alatzoglou, D., Li, C., Irving, M., Powell, D.J., and Coukos, G. (2015). T cells bearing a chimeric antigen receptor against prostate-specific membrane antigen mediate vascular disruption and result in tumor regression. Cancer Immunol Res 3, 68–84.
Schlenker, R., Olguín-Contreras, L.F., Leisegang, M., Schnappinger, J., Disovic, A., Rühland, S., Nelson, P.J., Leonhardt, H., Harz, H., Wilde, S., et al. (2017). Chimeric PD-1:28 receptor upgrades low-avidity T cells and restores effector function of tumor-infiltrating lymphocytes for adoptive cell therapy. Cancer Res 77, 3577–3590.
Schuster, S.J., Svoboda, J., Chong, E.A., Nasta, S.D., Mato, A.R., Anak, Ö., Brogdon, J.L., Pruteanu-Malinici, I., Bhoj, V., Landsburg, D., et al. (2017). Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 377, 2545–2554.
Siegler, E., Li, S., Kim, Y.J., and Wang, P. (2017). Designed ankyrin repeat proteins as Her2 targeting domains in chimeric antigen receptorengineered T cells. Human Gene Ther 28, 726–736.
Tang, X.Y., Sun, Y., Zhang, A., Hu, G.L., Cao, W., Wang, D.H., Zhang, B., and Chen, H. (2016). Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label phase I trial protocol. BMJ Open 6, e013904.
Tao, K., He, M., Tao, F., Xu, G., Ye, M., Zheng, Y., and Li, Y. (2018). Development of NKG2D-based chimeric antigen receptor-T cells for gastric cancer treatment. Cancer Chemother Pharmacol 82, 815–827.
Thistlethwaite, F.C., Gilham, D.E., Guest, R.D., Rothwell, D.G., Pillai, M., Burt, D.J., Byatte, A.J., Kirillova, N., Valle, J.W., Sharma, S.K., et al. (2017). The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother 66, 1425–1436.
Till, B.G., Jensen, M.C., Wang, J., Chen, E.Y., Wood, B.L., Greisman, H. A., Qian, X., James, S.E., Raubitschek, A., Forman, S.J., et al. (2008). Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20- specific T cells. Blood 112, 2261–2271.
Till, B.G., Jensen, M.C., Wang, J., Qian, X., Gopal, A.K., Maloney, D.G., Lindgren, C.G., Lin, Y., Pagel, J.M., Budde, L.E., et al. (2012). CD20- specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 119, 3940–3950.
Turtle, C.J., Hay, K.A., Hanafi, L.A., Li, D., Cherian, S., Chen, X., Wood, B., Lozanski, A., Byrd, J.C., Heimfeld, S., et al. (2017). Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. JCO 35, 3010–3020.
van der Stegen, S.J.C., Hamieh, M., and Sadelain, M. (2015). The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov 14, 499–509.
Velasquez, M.P., Szoor, A., Vaidya, A., Thakkar, A., Nguyen, P., Wu, M.F., Liu, H., and Gottschalk, S. (2017). CD28 and 41BB costimulation enhances the effector function of CD19-specific engager T cells. Cancer Immunol Res 5, 860–870.
Walker, A.J., Majzner, R.G., Zhang, L., Wanhainen, K., Long, A.H., Nguyen, S.M., Lopomo, P., Vigny, M., Fry, T.J., Orentas, R.J., et al. (2017). Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol Ther 25, 2189–2201.
Wang, J., Chen, S., Xiao, W., Li, W., Wang, L., Yang, S., Wang, W., Xu, L., Liao, S., Liu, W., et al. (2018). CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol 11, 7.
Wang, W., Qin, D.Y., Zhang, B.L., Wei, W., Wang, Y.S., and Wei, Y.Q. (2016a). Establishing guidelines for CAR-T cells: challenges and considerations. Sci China Life Sci 59, 333–339.
Wang, X., Popplewell, L.L., Wagner, J.R., Naranjo, A., Blanchard, M.S., Mott, M.R., Norris, A.P., Wong, C.L.W., Urak, R.Z., Chang, W.C., et al. (2016b). Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood 127, 2980–2990.
Wang, Y., Becker, D., Vass, T., White, J., Marrack, P., and Kappler, J.W. (2009). A conserved CXXC motif in CD3e is critical for T cell development and TCR signaling. PLoS Biol 7, e1000253.
Wang, Z., Guo, Y., and Han, W. (2017a). Current status and perspectives of chimeric antigen receptor modified T cells for cancer treatment. Protein Cell 8, 896–925.
Wang, Z., Wu, Z., Liu, Y., and Han, W. (2017b). New development in CAR-T cell therapy. J Hematol Oncol 10, 53.
Weng, J., Lai, P., Qin, L., Lai, Y., Jiang, Z., Luo, C., Huang, X., Wu, S., Shao, D., Deng, C., et al. (2018). A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J Hematol Oncol 11, 25.
Wu, Y., Jiang, S., and Ying, T. (2016). From therapeutic antibodies to chimeric antigen receptors (CARs): making better CARs based on antigen-binding domain. Expert Opin Biol Ther 16, 1469–1478.
Xiong, W., Chen, Y., Kang, X., Chen, Z., Zheng, P., Hsu, Y.H., Jang, J.H., Qin, L., Liu, H., Dotti, G., et al. (2018). Immunological synapse predicts effectiveness of chimeric antigen receptor cells. Mol Ther 26, 963–975.
You, F., Jiang, L., Zhang, B., Lu, Q., Zhou, Q., Liao, X., Wu, H., Du, K., Zhu, Y., Meng, H., et al. (2016). Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells. Sci China Life Sci 59, 386–397.
Zah, E., Lin, M.Y., Silva-Benedict, A., Jensen, M.C., and Chen, Y.Y. (2016). T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res 4, 498–508.
Zhang, Q., Tian, K., Xu, J., Zhang, H., Li, L., Fu, Q., Chai, D., Li, H., and Zheng, J. (2017). Synergistic effects of cabozantinib and EGFR-specific CAR-NK-92 cells in renal cell carcinoma. J Immunol Res 2017, 6915912.
Zhang, T., Wu, M.R., and Sentman, C.L. (2012). An NKp30-based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo. J Immunol 189, 2290–2299.
Zhang, Y., Zhang, W., Dai, H., Wang, Y., Shi, F., Wang, C., Guo, Y., Liu, Y., Chen, M., Feng, K., et al. (2016). An analytical biomarker for treatment of patients with recurrent B-ALL after remission induced by infusion of anti-CD19 chimeric antigen receptor T (CAR-T) cells. Sci China Life Sci 59, 379–385.
Zhao, Z., Condomines, M., van der Stegen, S.J.C., Perna, F., Kloss, C.C., Gunset, G., Plotkin, J., and Sadelain, M. (2015). Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28, 415–428.
Zheng, W., O’Hear, C.E., Alli, R., Basham, J.H., Abdelsamed, H.A., Palmer, L.E., Jones, L.L., Youngblood, B., and Geiger, T.L. (2018). PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia 32, 1157–1167.
Zhong, X.S., Matsushita, M., Plotkin, J., Riviere, I., and Sadelain, M. (2010). Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther 18, 413–420.
Acknowledgements
This work was supported by the grants from the National Key Research and Development Program of China (2016YFC1303501 and 2016YFC1303504) and the Science and Technology Planning Project of Beijing City (Z151100003915076) and the National Natural Science Foundation of China (81472612 and 81501682).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ti, D., Niu, Y., Wu, Z. et al. Genetic engineering of T cells with chimeric antigen receptors for hematological malignancy immunotherapy. Sci. China Life Sci. 61, 1320–1332 (2018). https://doi.org/10.1007/s11427-018-9411-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-018-9411-4