Abstract
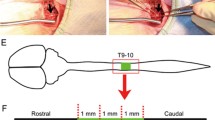
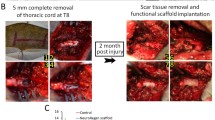
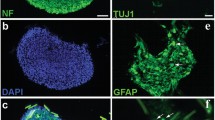
The present study aimed to explore the potential of the sodium hyaluronate-CNTF (ciliary neurotrophic factor) scaffold in activating endogenous neurogenesis and facilitating neural network re-formation after the adult rat spinal cord injury (SCI). After completely cutting and removing a 5-mm adult rat T8 segment, a sodium hyaluronate-CNTF scaffold was implanted into the lesion area. Dil tracing and immunofluorescence staining were used to observe the proliferation, differentiation and integration of neural stem cells (NSCs) after SCI. A planar multielectrode dish system (MED64) was used to test the electrophysiological characteristics of the regenerated neural network in the lesioned area. Electrophysiology and behavior evaluation were used to evaluate functional recovery of paraplegic rat hindlimbs. The Dil tracing and immunofluorescence results suggest that the sodium hyaluronate-CNTF scaffold could activate the NSCs originating from the spinal cord ependymal, and facilitate their migration to the lesion area and differentiation into mature neurons, which were capable of forming synaptic contact and receiving glutamatergic excitatory synaptic input. The MED64 results suggest that functional synapsis could be established among regenerated neurons as well as between regenerated neurons and the host tissue, which has been evidenced to be glutamatergic excitatory synapsis. The electrophysiology and behavior evaluation results indicate that the paraplegic rats’ sensory and motor functions were recovered in some degree. Collectively, this study may shed light on paraplegia treatment in clinics.
Similar content being viewed by others
References
Alto, L.T., Havton, L.A., Conner, J.M., Hollis II, E.R., Blesch, A., and Tuszynski, M.H. (2009). Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci 12, 1106–1113.
Arunkumar, M.J., Srinivasa, B.K., and Chandy, M.J. (2001). Motor and somatosensory evoked potentials in a primate model of experimental spinal cord injury. Neurol India 49, 219–224.
Basso, D.M., Beattie, M.S., and Bresnahan, J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12, 1–21.
Barnabé-Heider, F., Göritz, C., Sabelström, H., Takebayashi, H., Pfrieger, F.W., Meletis, K., and Frisén, J. (2010). Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7, 470–482.
Dickson, B.J., and Gilestro, G.F. (2006). Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol 22, 651–675.
de Almeida, L.P., Zala, D., Aebischer, P., and Déglon, N. (2001). Neuroprotective effect of a CNTF-expressing lentiviral vector in the quinolinic acid rat model of Huntington’s disease. Neurobiol Dis 8, 433–446.
Fink, A.J.P., Croce, K.R., Huang, Z.J., Abbott, L.F., Jessell, T.M., and Azim, E. (2014). Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 509, 43–48.
Gage, F.H. (2000). Mammalian neural stem cells. Science 287, 1433–1438.
Horn, E.M., Beaumont, M., Shu, X.Z., Harvey, A., Prestwich, G.D., Horn, K.M., Gibson, A.R., Preul, M.C., and Panitch, A. (2007). Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. J Neurosurg Spine 6, 133–140.
Liang, H., Paxinos, G., and Watson, C. (2012). The red nucleus and the rubrospinal projection in the mouse. Brain Struct Funct 217, 221–232.
Lowry, N.A., and Temple, S. (2007). Making human neurons from stem cells after spinal cord injury. PLoS Med 4, e48.
Li, X., Yang, Z., Zhang, A., Wang, T., and Chen, W. (2009). Repair of thoracic spinal cord injury by chitosan tube implantation in adult rats. Biomaterials 30, 1121–1132.
Luo, Y., Coskun, V., Liang, A., Yu, J., Cheng, L., Ge, W., Shi, Z., Zhang, K., Li, C., Cui, Y., Lin, H., Luo, D., Wang, J., Lin, C., Dai, Z., Zhu, H., Zhang, J., Liu, J., Liu, H., de Vellis, J., Horvath, S., Sun, Y.E., and Li, S. (2015). Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell 161, 1175–1186.
Maegele, M., Riess, P., Sauerland, S., Bouillon, B., Hess, S., McIntosh, T.K., Mautes, A., Brockmann, M., Koebke, J., Knifka, J., and Neugebauer, E.A.M. (2005). Characterization of a new rat model of experimental combined neurotrauma. Shock 23, 476–481.
Martin Bauknight, W., Chakrabarty, S., Hwang, B.Y., Malone, H.R., Joshi, S., Bruce, J.N., Sander Connolly, E., Winfree, C.J., Cunningham, M.G., Martin, J.H., and Haque, R. (2012). Convection enhanced drug delivery of BDNF through a microcannula in a rodent model to strengthen connectivity of a peripheral motor nerve bridge model to bypass spinal cord injury. J Clin Neurosci 19, 563–569.
Mladinic, M., and Nistri, A. (2013). Microelectrode arrays in combination with in vitro models of spinal cord injury as tools to investigate pathological changes in network activity: facts and promises. Front Neuroeng 3, 1–7.
Ming, G., and Song, H. (2005). Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28, 223–250.
Magloire, V., and Streit, J. (2009). Intrinsic activity and positive feedback in motor circuits in organotypic spinal cord slice cultures. Eur J Neurosci 30, 1487–1497.
Nakatomi, H., Kuriu, T., Okabe, S., Yamamoto, S., Hatano, O., Kawahara, N., Tamura, A., Kirino, T., and Nakafuku, M. (2002). Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110, 429–441.
Oka, H., Shimono, K., Ogawa, R., Sugihara, H., and Taketani, M. (1999). A new planar multielectrode array for extracellular recording: application to hippocampal acute slice. J Neurosci Methods 93, 61–67.
Poduslo, J.F., and Curran, G.L. (1996). Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Mol Brain Res 36, 280–286.
Sharp, J., Frame, J., Siegenthaler, M., Nistor, G., and Keirstead, HS. (2010). Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 28, 152–163.
Schlag, M.G., Hopf, R., and Redl, H. (2001). Serial recording of sensory, corticomotor, and brainstem-derived motor evoked potentials in the rat. Somatosens Motor Res 18, 106–116.
Shihabuddin, L.S., Horner, P.J., Ray, J., and Gage, F.H. (2000). Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci 20, 8727–8735.
Stankoff, B., Aigrot, M.S., Noel, F., Wattilliaux, A., Zalc, B., and Lubetzki, C. (2002). Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. J Neurosci 22, 9221–9227.
Tuinstra, H.M., Aviles, M.O., Shin, S., Holland, S.J., Zelivyanskaya, M.L., Fast, A.G., Ko, S.Y., Margul, D.J., Bartels, A.K., Boehler, R.M., Cummings, B.J., Anderson, A.J., and Shea, L.D. (2012). Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials 33, 1618–1626.
Wang, N., Zhang, S., Zhang, A.F., Yang, Z.Y., and Li, X.G. (2014). Sodium hyaluronate-CNTF gelatinous particles promote axonal growth, neurogenesis and functional recovery after spinal cord injury. Spinal Cord 52, 517–523.
Yang, Z., Zhang, A., Duan, H., Zhang, S., Hao, P., Ye, K., Sun, Y.E., and Li, X. (2015). NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci USA 112, 13354–13359.
Acknowledgements
This work was supported by the State Key Program of the National Natural Science Foundation of China (31130022, 31320103903, 31271037 & 31670988), the International Cooperation in Science and Technology Project of the Ministry of Science and Technology of China (2014DFA30640), the National Ministry of Education Special Fund for Excellent Doctoral Dissertation (201356), the Special Fund for Excellent Doctoral Dissertation of Beijing (20111000601), and the Special Funds for Beijing Base Construction & Talent Cultivation (171100002217066).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material







11427_2017_9217_MOESM8_ESM.doc
Application of the sodium hyaluronate-CNTF scaffolds in repairing adult rat spinal cord injury and facilitating neural network formation
Rights and permissions
About this article
Cite this article
Xie, Y., Song, W., Zhao, W. et al. Application of the sodium hyaluronate-CNTF scaffolds in repairing adult rat spinal cord injury and facilitating neural network formation. Sci. China Life Sci. 61, 559–568 (2018). https://doi.org/10.1007/s11427-017-9217-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-017-9217-2