Abstract
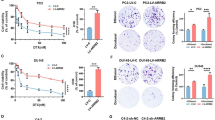
We previously demonstrated that matrine could inhibit the proliferating, migrating, as well as invading processes of both PC-3 and DU145 cells. However, the underlying molecular mechanisms have not yet been clearly defined. In this study, using various techniques such as high throughput sequencing technology, bioinformatics, quantitative real-time PCR, and immunoblot analysis, we aimed to understand whether matrine serves as a novel regulator of FOXO and PI3K-AKT signaling pathway. DU145 and PC-3 cell lines were cultured for 24 h in vitro. Cells were treated with either matrine or control serum for 48 h, followed by extraction of total RNA. The RNA was sequenced using HiSeq 2500 high-throughput sequencing platform (Illumina). A gene library was established and quality analysis of read data carried out. Integrated database from the website DAVID was used to analyze Gene Ontology (GO), and Kyoto encyclopedia of genes and genomes (KEGG) pathway of differential genes was used for pathway analysis, screening for fold differences of more than two times. The FOXO and PI3K-AKT signaling pathways were screened, and expression levels of mRNA and core protein detected by real-time PCR and immunoblotting, respectively. High throughput sequencing and GO analysis revealed that differentially expressed genes before and after treatment played an important role in cell metabolic process, growth process, anatomical structure formation, cellular component organization, and biological regulation. KEGG signal pathway analysis revealed that FOXO and PI3K-AKT signal pathways had a significant difference between before and after matrine-treated androgen-independent prostate cancer cells PC-3 and DU145. Real-time PCR showed that matrine treatment led to a significant increase in the expression levels of FOXO1A, FOXO3A, FOXO4, and FOXO6 in DU145 and PC-3 cells (P<0.01 or P<0.05), whereas the PI3K expression levels decreased (P<0.01). Similarly, immunoblotting revealed a significant increase (P<0.05) in the expression levels of FOXO1A FOXO3A, FOXO4, and FOXO6 in both PC-3 and DU145 cells, whereas PI3K expression levels decreased (P<0.05). Matrine had a broad regulating effect on the mRNA expression profiles of both PC-3 and DU145 cells. Matrine may inhibit cell proliferation, migration, as well as invasion, and induce apoptosis in both PC-3 and DU145 cells through FOXO and PI3K-AKT signaling pathways. Matrine could therefore be used as a complementary drug to present chemotherapeutic agents, for treating androgen-independent prostate cancer.
Similar content being viewed by others
References
Accili, D., and Arden, K.C. (2004). FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117, 421–426.
Anders, S., and Huber, W. (2010). Differential expression analysis for sequence count data. Genome Biol 11, R106.
Beelen, K., Hoefnagel, L.D.C., Opdam, M., Wesseling, J., Sanders, J., Vincent, A.D., van Diest, P.J., and Linn, S.C. (2014). PI3K/AKT/mTOR pathway activation in primary and corresponding metastatic breast tumors after adjuvant endocrine therapy. Int J Cancer 135, 1257–1263.
Brenkman, A.B., de Keizer, P.L.J., van den Broek, N.J.F., Jochemsen, A.G., and Burgering, B.M.T. (2008). Mdm2 induces mono-ubiquitination of FOXO4. PLoS ONE 3, e2819.
Calnan, D.R., and Brunet, A. (2008). The FoxO code. Oncogene 27, 2276–2288.
Chen, W., Zheng, R., Baade, P.D., Zhang, S., Zeng, H., Bray, F., Jemal, A., Yu, X.Q., and He, J. (2016). Cancer statistics in China, 2015. CA Cancer J Clin 66, 115–132.
Fei, M., Zhao, Y., Wang, Y., Lu, M., Cheng, C., Huang, X., Zhang, D., Lu, J., He, S., and Shen, A. (2009). Low expression of Foxo3a is associated with poor prognosis in ovarian cancer patients. Cancer Invest 27, 52–59.
Guerin, M., Qian, C., Zhong, Q., Cui, Q., Guo, Y., Bei, J., Shao, J., Zhu, X., Huang, W., Wu, J., Liu, R., Liu, Q., Wang, J., Jia, W., Zheng, X., and Zeng, Y. (2016). Translational oncology toward benefiting cancer patients: the Sun Yat-sen University Cancer Center experience. Sci China Life Sci 59, 1057–1062.
Hagenbuchner, J., Kuznetsov, A., Hermann, M., Hausott, B., Obexer, P., and Ausserlechner, M.J. (2012). FOXO3-induced reactive oxygen species are regulated by BCL2L11 (Bim) and SESN3. J Cell Sci 125, 1191–1203.
Halacli, S.O., and Dogan, A.L. (2015). FOXP1 regulation via the PI3K/Akt/p70S6K signaling pathway in breast cancer cells. Oncol Lett 9, 1482–1488.
Huang, H., and Tindall, D.J. (2007). Dynamic FoxO transcription factors. J Cell Sci 120, 2479–2487.
Klotz, L.O., Sánchez-Ramos, C., Prieto-Arroyo, I., Urbánek, P., Steinbrenner, H., and Monsalve, M. (2015). Redox regulation of FoxO transcription factors. Redox Biol 6, 51–72.
Kumar, N., Crocker, T., Smith, T., Pow-Sang, J., Spiess, P.E., Connors, S., Chornukur, G., Dickinson, S.I., Bai, W., Williams, C.R., Salup, R., and Fu, W. (2012). Prostate cancer chemoprevention targeting high risk populations: model for trial design and outcome measures. J Cancer Sci Ther 2011, pii: 007.
Lai, J.P., He, X.W., Jiang, Y., and Chen, F. (2003). Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal Bioanal Chem 375, 264–269.
Li, Q., Lai, Y., Wang, C., Xu, G., He, Z., Shang, X., Sun, Y., Zhang, F., Liu, L., and Huang, H. (2016). Matrine inhibits the proliferation, invasion and migration of castration-resistant prostate cancer cells through regulation of the NF-κB signaling pathway. Oncol Rep 35, 375–381.
Liu, J.Y., Hu, J.H., Zhu, Q.G., Li, F.Q., Wang, J., and Sun, H.J. (2007). Effect of matrine on the expression of substance P receptor and inflammatory cytokines production in human skin keratinocytes and fibroblasts. Int Immunopharmacol 7, 816–823.
Medema, R.H., Kops, G.J.P.L., Bos, J.L., and Burgering, B.M. (2000). AFXlike Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404, 782–787.
Miller, K.D., Siegel, R.L., Lin, C.C., Mariotto, A.B., Kramer, J.L., Rowland, J.H., Stein, K.D., Alteri, R., and Jemal, A. (2016). Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66, 271–289.
Muranen, T., Selfors, L.M., Worster, D.T., Iwanicki, M.P., Song, L., Morales, F.C., Gao, S., Mills, G.B., and Brugge, J.S. (2012). Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell 21, 227–239.
Newton, R.H., and Turka, L.A. (2012). Regulation of T cell homeostasis and responses by pten. Front Immun 3, 151.
Plas, D.R., and Thompson, C.B. (2003). Akt Activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem 278, 12361–12366.
Quon, H., and Loblaw, D.A. (2010). Androgen deprivation therapy for prostate cancer-review of indications in 2010. Curr Oncol 17 Suppl 2, S38–S44.
Salih, D.A.M., and Brunet, A. (2008). FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol 20, 126–136.
Siegel, R.L., Miller, K.D., and Jemal, A. (2016). Cancer statistics, 2016. CA Cancer J Clin 66, 7–30.
Singh, A., Plati, J., and Khosravi-Far, R. (2011). Harnessing the tumor suppressor function of FOXO as an alternative therapeutic approach in cancer. Curr Drug Targets 12, 1311–1321.
Trapnell, C., Roberts, A., Goff, L., Pertea, G., Kim, D., Kelley, D.R., Pimentel, H., Salzberg, S.L., Rinn, J.L., and Pachter, L. (2012). Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7, 562–578.
van der Vos, K.E., and Coffer, P.J. (2011). The extending network of FOXO transcriptional target genes. Antioxid Redox Signal 14, 579–592.
Wang, L., Feng, Z., Wang, X., Wang, X., and Zhang, X. (2010). DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138.
Wang, Y., Zhou, Y., and Graves, D.T. (2014). FOXO transcription factors: their clinical significance and regulation. BioMed Res Int 2014, 1–13.
Wilk, A., Urbanska, K., Grabacka, M., Mullinax, J., Marcinkiewicz, C., Impastato, D., Estrada, J.J., and Reiss, K. (2012). Fenofibrate-induced nuclear translocation of FoxO3A triggers Bim-mediated apoptosis in glioblastoma cells in vitro. Cell Cycle 11, 2660–2671.
Xie, L., Ushmorov, A., Leithäuser, F., Guan, H., Steidl, C., Färbinger, J., Pelzer, C., Vogel, M.J., Maier, H.J., Gascoyne, R.D., Möller, P., and Wirth, T. (2012). FOXO1 is a tumor suppressor in classical Hodgkin lymphoma. Blood 119, 3503–3511.
Zeng, C.W., Wang, W.T., Yu, X.B., Yang, L.J., Chen, S.H., and Li, Y.Q. (2015). Pathways related to PMA-differentiated THP1 human monocytic leukemia cells revealed by RNA-Seq. Sci China Life Sci 58, 1282–1287.
Zhang, J., Li, Y., Chen, X., Liu, T., Chen, Y., He, W., Zhang, Q., and Liu, S. (2011). Autophagy is involved in anticancer effects of matrine on SGC-7901 human gastric cancer cells. Oncol Rep 26, 115–124.
Zhang, Y., Tseng, C.C., Tsai, Y.L., Fu, X., Schiff, R., and Lee, A.S. (2013). Cancer cells resistant to therapy promote cell surface relocalization of GRP78 which complexes with PI3K and enhances PI(3,4,5)P3 production. PLoS ONE 8, e80071.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81472382), the National Natural Science Foundation of China for Young Scientists (81101947), the Guangdong Province Natural Science Foundation (2014A030313079), the Fundamental Research Funds for the Central Universities (14ykpy19), Guangdong Province Science and Technology for Social Development Project (2013B021800107), Guangzhou City in 2015 scientific research projects (7415600066401 to Hai Huang).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Q., Huang, H., He, Z. et al. Regulatory effects of antitumor agent matrine on FOXO and PI3K-AKT pathway in castration-resistant prostate cancer cells. Sci. China Life Sci. 61, 550–558 (2018). https://doi.org/10.1007/s11427-016-9050-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-016-9050-6