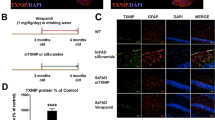
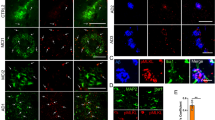
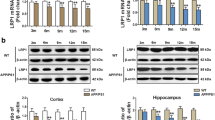
Abstract
Accumulating evidence suggests that β-amyloid (Aβ)-induced neuroinflammation plays a prominent and early role in Alzheimer’s disease (AD). In this study, we demonstrated that Presenilin 2 (PS2) deficiency facilitates Aβ-induced neuroinflammation and injury by upregulating P2X7 expression both in vitro and in vivo. PS2 knockout mice demonstrated increased cognitive impairments and cerebral injury. PS2 deficiency increased the expression of P2X7 both in neurons and microglial cells. Furthermore, extracellular ATP also increased in both Aβ-treated and untreated PS2 knockout microglial cells. Notably, Aβ-induced classical proinflammatory cytokines such as IL-1β, IL-1α and TNF-α were increased in PS2 knockout microglial cells, suggesting a potential role for PS2 in the regulation of neuroinflammation. The expression of P2X7 clearly increased in PS2 knockdown BV2 cells. Consistent with in vivo data, Aβ-induced IL-1β production was also clearly enhanced in PS2 knockdown BV2 cells. Additionally, expression of the transcription factor Sp1 was increased in PS2 knockdown cells. When we treated PS2 knockdown cells with the specific Sp1 inhibitor MIT, we observed that enhanced P2X7 expression was significantly rescued. Taken together, these data suggests that PS2 plays a protective role during Aβ-induced neuroinflammation and injury through down-regulation of P2X7 expression.
Similar content being viewed by others
References
Agrawal, V., Sawhney, N., Hickey, E., and McCarthy, J.V. (2016). Loss of presenilin 2 function is associated with defective LPS-mediated innate immune responsiveness. Mol Neurobiol 53, 3428–3438.
Akiyama, H., Barger, S., Barnum, S., Bradt, B., Bauer, J., Cole, G.M., Cooper, N.R., Eikelenboom, P., Emmerling, M., Fiebich, B.L., Finch, C.E., Frautschy, S., Griffin, W.S., Hampel, H., Hull, M., Landreth, G., Lue, L., Mrak, R., Mackenzie, I.R., McGeer, P.L., O’Banion, M.K., Pachter, J., Pasinetti, G., Plata-Salaman, C., Rogers, J., Rydel, R., Shen, Y., Streit, W., Strohmeyer, R., Tooyoma, I., Van, M.F.L., Veerhuis, R., Walker, D., Webster, S., Wegrzyniak, B., Wenk, G., and Wyss-Coray, T. (2000). Inflammation and Alzheimer’s disease. Neurobiol Aging 21, 383–421.
Beglopoulos, V., Sun, X., Saura, C.A., Lemere, C.A., Kim, R.D., and Shen, J. (2004). Reduced ß-amyloid production and increased inflammatory responses in presenilin conditional knock-out mice. J Biol Chem 279, 46907–46914.
Bhattacharya, A., and Biber, K. (2016). The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 64, 1772–1787.
Busciglio, J., Gabuzda, D.H., Matsudaira, P., and Yankner, B.A. (1993). Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci USA 90, 2092–2096.
Canevelli, M., Piscopo, P., Talarico, G., Vanacore, N., Blasimme, A., Crestini, A., Tosto, G., Troili, F., Lenzi, G.L., Confaloni, A., and Bruno, G. (2014). Familial Alzheimer’s disease sustained by presenilin 2 mutations: systematic review of literature and genotype-phenotype correlation. Neurosci Biobehav Rev 42, 170–179.
Citron, B.A., Dennis, J.S., Zeitlin, R.S., and Echeverria, V. (2008). Transcription factor Sp1 dysregulation in Alzheimer’s disease. J Neurosci Res 86, 2499–2504.
Citron, B.A., Saykally, J.N., Cao, C., Dennis, J.S., Runfeldt, M., and Arendash, G.W. (2015). Transcription factor Sp1 inhibition, memory, and cytokines in a mouse model of Alzheimer’s disease. Am J Neurodegener Dis 4, 40–48.
Collo, G., Neidhart, S., Kawashima, E., Kosco-Vilbois, M., North, R.A., and Buell, G. (1997). Tissue distribution of the P2X7 receptor. Neuropharmacology 36, 1277–1283.
Davalos, D., Grutzendler, J., Yang, G., Kim, J.V., Zuo, Y., Jung, S., Littman, D.R., Dustin, M.L., and Gan, W.B. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8, 752–758.
Diaz-Hernandez, J.I., Gomez-Villafuertes, R., León-Otegui, M., Hontecillas-Prieto, L., Del Puerto, A., Trejo, J.L., Lucas, J.J., Garrido, J.J., Gualix, J., Miras-Portugal, M.T., and Diaz-Hernandez, M. (2012). In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3ß and secretases. Neurobiol Aging 33, 1816–1828.
Feng, R., Wang, H., Wang, J., Shrom, D., Zeng, X., and Tsien, J.Z. (2004). Forebrain degeneration and ventricle enlargement caused by double knockout of Alzheimer’s presenilin-1 and presenilin-2. Proc Natl Acad Sci USA 101, 8162–8167.
Fernández, J.A., Rojo, L., Kuljis, R.O., and Maccioni, R.B. (2008). The damage signals hypothesis of Alzheimer’s disease pathogenesis. J Alzheimer’s Dis 14, 329–333.
Ferrari, D., Pizzirani, C., Adinolfi, E., Lemoli, R.M., Curti, A., Idzko, M., Panther, E., and Di Virgilio, F. (2006). The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176, 3877–3883.
Fillenbaum, G.G., van Belle, G., Morris, J.C., Mohs, R.C., Mirra, S.S., Davis, P.C., Tariot, P.N., Silverman, J.M., Clark, C.M., Welsh-Bohmer, K.A., and Heyman, A. (2008). Consortium to establish a registry for Alzheimer’s disease (CERAD): the first twenty years. Alzheimer’s Dementia 4, 96–109.
García-Huerta, P., Díaz-Hernandez, M., Delicado, E.G., Pimentel-Santillana, M., Miras-Portugal, M.T., and Gómez-Villafuertes, R. (2012). The specificity protein factor Sp1 mediates transcriptional regulation of P2X7 receptors in the nervous system. J Biol Chem 287, 44628–44644.
Gombault, A., Baron, L., and Couillin, I. (2013). ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol 3, 414.
Haaßs, C., Hung, A.Y., Schloßsmacher, M.G., Oltersdorf, T., Teplow, D.B., and Selkoe, D.J. (1993). Normal cellular processing of the ß-amyloid precursor protein results in the secretion of the amyloid ß peptide and related moleculesa. Ann New York Acad Sci 695, 109–116.
Heneka, M.T., Golenbock, D.T., and Latz, E. (2015). Innate immunity in Alzheimer’s disease. Nat Immunol 16, 229–236.
Herreman, A., Hartmann, D., Annaert, W., Saftig, P., Craessaerts, K., Serneels, L., Umans, L., Schrijvers, V., Checler, F., Vanderstichele, H., Baekelandt, V., Dressel, R., Cupers, P., Huylebroeck, D., Zwijsen, A., Van Leuven, F., and De Strooper, B. (1999). Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci USA 96, 11872–11877.
Hu, X., Wang, T., and Jin, F. (2016). Alzheimer’s disease and gut microbiota. Sci China Life Sci 59, 1006–1023.
Jiang, X., Zhang, D., Shi, J., Chen, Y., Zhang, P., and Mei, B. (2009). Increased inflammatory response both in brain and in periphery in presenilin 1 and presenilin 2 conditional double knock-out mice. J Alzheimer’s Dis 18, 515–523.
Kovacs, D.M., Fausett, H.J., Page, K.J., Kim, T.W., Moir, R.D., Merriam, D.E., Hollister, R.D., Hallmark, O.G., Mancini, R., Felsenstein, K.M., Hyman, B.T., Tanzi, R.E., and Wasco, W. (1996). Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat Med 2, 224–229.
Lee, H.G., Won, S.M., Gwag, B.J., and Lee, Y.B. (2011). Microglial P2X7 eceptor expression is accompanied by neuronal damage in the cerebral cortex of the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Exp Mol Med 43, 7.
Mandrekar-Colucci, S., and Landreth, G.E. (2010). Microglia and inflammation in Alzheimer’s disease. CNS Neurol Disord Drug Targets 9, 156–167.
McLarnon, J.G., Ryu, J.K., Walker, D.G., and Choi, H.B. (2006). Upregulated expreßsion of purinergic P2X7 receptor in Alzheimer disease and amyloid-ß peptide-treated microglia and in peptide-injected rat hippocampus. J Neuropathol Exp Neurol 65, 1090–1097.
Morales, I., Farías, G., and Maccioni, R.B. (2010). Neuroimmunomodulation in the pathogenesis of Alzheimer’s disease. Neuroimmunomodulation 17, 202–204.
Morales, I., Guzmn-Mart-nez, L., Cerda-Troncoso, C.Ã., FarÃ-as, G.A., and Maccioni, R.B. (2014). Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci 8, 112.
Parajuli, B., Sonobe, Y., Horiuchi, H., Takeuchi, H., Mizuno, T., and Suzumura, A. (2013). Oligomeric amyloid ß induces IL-1ß processing via production of ROS: implication in Alzheimer’s disease. Cell Death Dis 4, e975.
Prince, M., Wimo, A., Guerchet, M., Ali, G.C., Wu, Y.T., and Prina, M. (2015). World Alzheimer Report 2015: The Global Impact of Dementia. (London: Alzheimer’s Disease International (ADI)).
Rodríguez, J.J., Olabarria, M., Chvatal, A., and Verkhratsky, A. (2009). Astroglia in dementia and Alzheimer’s disease. Cell Death Differ 16, 378–385.
Saijo, K., and Glass, C.K. (2011). Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11, 775–787.
Sanz, J.M., Chiozzi, P., Ferrari, D., Colaianna, M., Idzko, M., Falzoni, S., Fellin, R., Trabace, L., and Di Virgilio, F. (2009). Activation of microglia by amyloid ß requires P2X7 receptor expression. J Immunol 182, 4378–4385.
Saura, C.A., Choi, S.Y., Beglopoulos, V., Malkani, S., Zhang, D., Rao, B.S.S., Chattarji, S., Kelleher, R.J., Kandel, E.R., Duff, K., Kirkwood, A., and Shen, J. (2004). Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42, 23–36.
Saura, J., Tusell, J.M., and Serratosa, J. (2003). High-yield isolation of murine microglia by mild trypsinization. Glia 44, 183–189.
Selkoe, D.J., and Wolfe, M.S. (2007). Presenilin: running with scissors in the membrane. Cell 131, 215–221.
Shen, J., and Kelleher, R.J. (2007). The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci USA 104, 403–409.
Shieh, C.H., Heinrich, A., Serchov, T., van Calker, D., and Biber, K. (2014). P2X7-dependent, but differentially regulated release of IL-6, CCL2, and TNF-a in cultured mouse microglia. Glia 62, 592–607.
Skaper, S.D., Debetto, P., and Giusti, P. (2010). The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J 24, 337–345.
Smolarkiewicz, M., Skrzypczak, T., and Wojtaszek, P. (2013). The very many faces of presenilins and the secretase complex. Protoplasma 250, 997–1011.
Supnet, C., and Bezprozvanny, I. (2011). Presenilins function in ER calcium leak and Alzheimer’s disease pathogenesis. Cell Calcium 50, 303–309.
Takenouchi, T., Sekiyama, K., Sekigawa, A., Fujita, M., Waragai, M., Sugama, S., Iwamaru, Y., Kitani, H., and Hashimoto, M. (2010). P2X7 receptor signaling pathway as a therapeutic target for neurodegenerative diseases. Arch Immunol Ther Exp 58, 91–96.
Verderio, C., and Matteoli, M. (2001). ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN. J Immunol 166, 6383–6391.
Villa, C., Ridolfi, E., Fenoglio, C., Ghezzi, L., Vimercati, R., Clerici, F., Marcone, A., Gallone, S., Serpente, M., Cantoni, C., Bonsi, R., Cioffi, S., Cappa, S., Franceschi, M., Rainero, I., Mariani, C., Scarpini, E., and Galimberti, D. (2013). Expression of the transcription factor Sp1 and its regulatory hsa-miR-29b in peripheral blood mononuclear cells from patients with Alzheimer’s disease. J Alzheimer’s Dis 35, 487–494.
Vorhees, C.V., and Williams, M.T. (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1, 848–858.
Weggen, S., Eriksen, J.L., Das, P., Sagi, S.A., Wang, R., Pietrzik, C.U., Findlay, K.A., Smith, T.E., Murphy, M.P., Bulter, T., Kang, D.E., Marquez-Sterling, N., Golde, T.E., and Koo, E.H. (2001). A subset of NSAIDs lower amyloidogenic Aß42 independently of cyclooxygenase activity. Nature 414, 212–216.
Wes, P.D., Sayed, F.A., Bard, F., and Gan, L. (2016). Targeting microglia for the treatment of Alzheimer’s disease. Glia 64, 1710–1732.
Wolfe, M.S. (2007). When loßs is gain: reduced presenilin proteolytic function leads to increased Aß42/Aß40. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep 8, 136–140.
Wolfe, M.S. (2013). Toward the structure of presenilin/secretase and presenilin homologs. Biochim Biophys Acta 1828, 2886–2897.
Woods, L.T., Ajit, D., Camden, J.M., Erb, L., and Weisman, G.A. (2016). Purinergic receptors as potential therapeutic targets in Alzheimer’s disease. Neuropharmacology 104, 169–179.
Yang, D., He, Y., Muñoz-Planillo, R., Liu, Q., and Núñez, G. (2015). Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43, 923–932.
Yeo, J.F., Ong, W.Y., Ling, S.F., and Farooqui, A.A. (2004). Intracerebroventricular injection of phospholipases A2 inhibitors modulates allodynia after facial carrageenan injection in mice. Pain 112, 148–155.
Zhang, F., and Jiang, L.L. (2015). Neuroinflammation in Alzheimer’s disease. Neuropsychiatr Dis Treat 11, 243.
Zou, J., Vetreno, R.P., and Crews, F.T. (2012). ATP-P2X7 receptor signaling controls basal and TNFa-stimulated glial cell proliferation. Glia 60, 661–673.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31470040, 31570896, 81172816, 81672811), Doctoral Fund of Ministry of Education of China (20130076110013), and Science and Technology Commission of Shanghai Municipality (15JC1401500).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Contributed equally to this work
Rights and permissions
About this article
Cite this article
Qin, J., Zhang, X., Wang, Z. et al. Presenilin 2 deficiency facilitates Aβ-induced neuroinflammation and injury by upregulating P2X7 expression. Sci. China Life Sci. 60, 189–201 (2017). https://doi.org/10.1007/s11427-016-0347-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-016-0347-4