Abstract
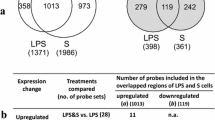
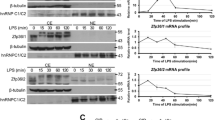
We have proposed that analysis of ribosome-loaded mRNAs (i.e., the translatome) is useful for elucidation of pharmacological effects of phytocompounds in immune cells, regarding the involvement of post-transcriptional regulation mechanisms. In the present study, we compared the effects of pachymic acid from Poria cocos fungus and moronic acid from propolis with those of hydrocortisone on the translatomes of THP-1 macrophages exposed to bacterial lipopolysaccharide (LPS) to find clues to their biological effects. Polysome-associated RNAs collected from cells treated for 3 h with LPS plus each of the compounds were analyzed by DNA microarray followed by analyses of pathways/gene ontologies (GO). Upregulated mRNAs in enriched pathways that were found to contain AUUUA (AU)-rich motifs were checked by real-time PCR, and expression of candidate RNA-binding proteins stabilizing/destabilizing such AU-rich mRNAs was checked by Western blotting. The numbers of upregulated and downregulated genes (fold-changes ± 2.0 versus vehicle-control) were, respectively, 209 and 125 for moronic acid, 23 and 2 for pachymic acid, and 214 and 59 for hydrocortisone treatment. Overlapping with hydrocortisone treatment for upregulation were 158 genes in moronic acid and 17 in pachymic acid treatment; of these, 16 overlapped within all treatments (C-X-C motif chemokine ligands, interferon-induced protein with tetratricopeptide repeats, etc.). Pathway analyses showed GO enrichments such as ‘immune response’, ‘receptor binding’, ‘extracellular space’ etc. The pachymic acid-upregulated mRNAs (highly overlapped with the other 2 treatments) showed the presence of signal peptides and AU-rich motifs, suggesting regulation by AU-rich element (ARE)-binding proteins. The expression of ARE-binding protein HuR/ELAV-1 was increased by the 3 compounds, and AUF1/hnRNP D was decreased by pachymic acid. These results suggested that pachymic acid and moronic acid effects may involve as yet unknown post-transcriptional modulation via ARE-binding proteins resembling that of glucocorticoids.





Similar content being viewed by others
Abbreviations
- AU-rich:
-
Adenylate-uridylate (AUUUA)-rich
- ARE:
-
AU-rich element
- AUF1/hnRNP D:
-
AU-rich element RNA-binding protein 1/heterogeneous nuclear ribonucleoprotein D
- DMSO:
-
Dimethylsulfoxide
- FDR:
-
False discovery rate
- GO:
-
Gene ontology
- HuR/ELAV-like protein 1:
-
Hu-antigen R/embryonic lethal, abnormal vision, Drosophila-like protein 1
- LPS:
-
Lipopolysaccharide
- PPI:
-
Protein-protein interaction
- RT-qPCR:
-
Reverse-transcription quantitative real-time PCR
- TNF-α:
-
Tumor necrosis factor-alpha
References
Yasukawa K, Sun Y, Kitanaka S, Tomizawa N, Miura M, Motohashi S (2008) Inhibitory effect of the rhizomes of Alpinia officinarum on TPA-induced inflammation and tumor promotion in two-stage carcinogenesis in mouse skin. J Nat Med 62:374–378
Sun Y, Tabata K, Matsubara H, Kitanaka S, Suzuki T, Yasukawa K (2008) New cytotoxic diarylheptanoids from the rhizomes of Alpinia officinarum. Planta Med 74:427–431
Tabata K, Yamazaki Y, Okada M, Fukumura K, Shimada A, Sun Y, Yasukawa K, Suzuki T (2009) Diarylheptanoids derived from Alpinia officinarum induce apoptosis, S-phase arrest and differentiation in human neuroblastoma cells. Anticancer Res 29:4981–4988
Sawamura R, Sun Y, Yasukawa K, Shimizu T, Watanabe W, Kurokawa M (2010) Antiviral activities of diarylheptanoids against influenza virus in vitro. J Nat Med 64:117–120
Sawamura R, Shimizu T, Sun Y, Yasukawa K, Miura M, Toriyama M, Motohashi S, Watanabe W, Konno K, Kurokawa M (2010) In vitro and in vivo anti-influenza virus activity of diarylheptanoids isolated from Alpinia officinarum. Antivir Chem Chemother 21:33–41
Konno K, Sawamura R, Sun Y, Yasukawa K, Shimizu T, Watanabe W, Kato M, Yamamoto R, Kurokawa M (2011) Antiviral activities of diarylheptanoids isolated from Alpinia officinarum against respiratory syncytial virus, poliovirus, measles virus, and herpes simplex virus type 1 in vitro. Nat Prod Commun 6:1881–1884
Konno K, Miura M, Toriyama M, Motohashi S, Sawamura R, Watanabe W, Yoshida H, Kato M, Yamamoto R, Yasukawa K (2013) Antiviral activity of diarylheptanoid stereoisomers against respiratory syncytial virus in vitro and in vivo. J Nat Med 67:773–781
Kakegawa T, Miyazaki A, Yasukawa K (2016) Anti-inflammatory effects of alpinone 3-acetate from Alpinia japonica seeds. J Nat Med 70:653–660
Kakegawa T, Takase S, Masubuchi E, Yasukawa K (2014) Diarylheptanoids from Alpinia officinarum cause distinct but overlapping effects on the translatome of B lymphoblastoid cells. Evid Based Complement Alternat Med. https://doi.org/10.1155/2014/204797
Yoshida LS, Kakegawa T, Yuda Y, Takano-Ohmuro H (2017) Shikonin changes the lipopolysaccharide-induced expression of inflammation-related genes in macrophages. J Nat Med 71:723–734
Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M (2011) Global quantification of mammalian gene expression control. Nature 473:337–342
Tebaldi T, Re A, Viero G, Pegoretti I, Passerini A, Blanzieri E, Quattrone A (2012) Widespread uncoupling between transcriptome and translatome variations after a stimulus in mammalian cells. BMC Genomics 13:220. https://doi.org/10.1186/1471-2164-13-220
Schimmer BP, Funder JW (2011) ACTH, adrenal steroids, and pharmacology of the adrenal cortex. In: Brunton LL, Chabner BA, Knollmann BC (eds) Goodman & Gilman’s the pharmacological basis of therapeutics, 12th edn. McGraw Hill Medical, New York, pp 1209–1235
Buttgereit F, Burmester GR, Lipworth BJ (2005) Optimised glucocorticoid therapy: the sharpening of an old spear. Lancet 365:801–803
Lu L, Li T, Williams G, Petit E, Borowsky M, Walker WA (2011) Hydrocortisone induces changes in gene expression and differentiation in immature human enterocytes. Am J Physiol Gastrointest Liver Physiol 300:G425–432
Ishmael FT, Fang X, Houser KR, Pearce K, Abdelmohsen K, Zhan M, Gorospe M, Stellato C (2011) The human glucocorticoid receptor as an RNA-binding protein: global analysis of glucocorticoid receptor-associated transcripts and identification of a target RNA motif. J Immnunol 86:1189–1198
Stellato C (2012) Posttranscriptional gene regulation: novel pathways for glucocorticoids’ anti-inflammatory action. Transl Med UniSa 3:67–73
Ignatchenko V, Ignatchenko A, Sinha A, Boutros PC, Kislinger T (2015) VennDIS: a JavaFX-based Venn and Euler diagram software to generate publication quality figures. Proteomics 15:1239–1244
Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR (2002) GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet 31:19–20
Pico AR, Kelder T, van Iersel MP, Hanspers K, Conklin BR, Evelo C (2008) WikiPathways: pathway editing for the people. PLoS Biol 6:e184. https://doi.org/10.1371/journal.pbio.0060184
Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP et al (2015) STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43(Database issue):D447–D452
Tai T, Akita Y, Kinoshita K, Koyama K, Takahashi K, Watanabe K (1995) Anti-emetic principles of Poria cocos. Planta Med 61:527–530
Li FF, Yuan Y, Liu Y, Wu QQ, Jiao R, Yang Z, Zhou MQ, Tang QZ (2015) Pachymic acid protects H9c2 cardiomyocytes from lipopolysaccharide-induced inflammation and apoptosis by inhibiting the extracellular signal-regulated kinase 1/2 and p38 pathways. Mol Med Rep 12:2807–2813
Ma J, Liu J, Lu C, Cai D (2015) Pachymic acid induces apoptosis via activating ROS-dependent JNK and ER stress pathways in lung cancer cells. Cancer Cell Int 15:78. https://doi.org/10.1186/s12935-015-0230-0
Kaminaga T, Yasukawa K, Kanno H, Tai T, Nunoura Y, Takido M (1996) Inhibitory effects of lanostane-type triterpene acids, the components of Poria cocos, on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology 53:382–385
Gapter L, Wang Z, Glinski J, Ng KY (2005) Induction of apoptosis in prostate cancer cells by pachymic acid from Poria cocos. Biochem Biophys Res Commun 332:1153–1161
Ling H, Zhang Y, Ng KY, Chew EH (2011) Pachymic acid impairs breast cancer cell invasion by suppressing nuclear factor-κB-dependent matrix metalloproteinase-9 expression. Breast Cancer Res Treat 126:609–620
Cheng S, Swanson K, Eliaz I, McClintick JN, Sandusky GE, Sliva D (2015) Pachymic acid inhibits growth and induces apoptosis of pancreatic cancer in vitro and in vivo by targeting ER stress. PLoS One 10:e0122270. https://doi.org/10.1371/journal.pone.0122270
Gao AH, Zhang L, Chen X, Chen Y, Xu ZZ, Liu YN, Zhang H (2015) Inhibition of ovarian cancer proliferation and invasion by pachymic acid. Int J Clin Exp Pathol 8:2235–2241
Wen H, Wu Z, Hu H, Wu Y, Yang G, Lu J, Yang G, Guo G, Dong Q (2017) The anti-tumor effect of pachymic acid on osteosarcoma cells by inducing PTEN and caspase 3/7-dependent apoptosis. J Nat Med. https://doi.org/10.1007/s11418-017-1117-2
Ito J, Chang FR, Wang HK, Park YK, Ikegaki M, Kilgore N, Lee KH (2001) Anti-AIDS agents. 48. (1) Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis. J Nat Prod 64:1278–1281
Gehrke IT, Neto AT, Pedroso M, Mostardeiro CP, Da Cruz IB, Silva UF, Ilha V, Dalcol II, Morel AF (2013) Antimicrobial activity of Schinus lentiscifolius (Anacardiaceae). J Ethnopharmacol 148:486–491
Ramirez-Espinosa JJ, Rios MY, Lopez-Martinez S, Lopez-Vallejo F, Medina-Franco JL, Paoli P, Camici G, Navarrete-Vazquez G, Ortiz-Andrade R, Estrada-Soto S (2011) Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP-1B: in vitro, in silico, and in vivo approaches. Eur J Med Chem 46:2243–2251
Fan J, Ishmael FT, Fang X, Myers A, Cheadle C, Huang SK, Atasoy U, Gorospe M, Stellato C (2011) Chemokine transcripts as targets of the RNA-binding protein HuR in human airway epithelium. J Immunol 186:2482–2494
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kakegawa, T., Yoshida, L.S., Takada, M. et al. Comparison of the effects of pachymic acid, moronic acid and hydrocortisone on the polysome loading of RNAs in lipopolysaccharide-treated THP-1 macrophages. J Nat Med 73, 190–201 (2019). https://doi.org/10.1007/s11418-018-1260-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-1260-4