Abstract
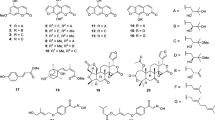
The methanolic extracts from the peels of Citrus limon were found to show antimutagenic effects against 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole, and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the Ames test. From the methanolic extracts, four new coumarins (wakayamalimonol A–D) and a new furanocoumarin (wakayamalimonol E) were isolated together with fifteen known compounds. The absolute stereostructures of the new compounds were determined by chemical synthesis and the modified Mosher’s method. Among the isolated constituents, coumarins, furanocoumarins, and limonoids showed antimutagenic effects in the Ames test. One of the major constituent, limonin, showed significant antimutagenic effects against mitomycinC and PhIP in the micronucleus test in vivo.




Similar content being viewed by others
References
Bruno L, Spadafora ND, Iaria D, Chiappetta A, Bitonti MB (2014) Developmental stimuli and stress factors affect expression of ClGLP1, an emerging allergen-related gene in Citrus limon. Plant Physiol Biochem 79:31–40
Campelo LM, Goncalves FC, Feitosa CM, Freitas RM (2011) Antioxidant activity of Citrus limon essential oil in mouse hippocampus. Pharm Biol 49:709–715
Shimizu S, Miyamoto S, Fujii G, Onuma W, Ozaki Y, Fujimoto K, Yano T, Mutoh M (2015) Suppression of intestinal carcinogenesis in Apc-mutant mice by limonin. J Clin Nutr 57:39–43
Matsumoto T, Nishikawa T, Furukawa A, Itano S, Tamura Y, Hasei T, Watanabe T (2017) Antimutagenic effects of polymethoxy flavonoids of Citrus unshiu. Nat Prod Commun 12:23–26
Liu DP, Luo Q, Wang GH, Xu Y, Zhang XK, Chen QC, Chen HF (2011) Furocoumarin derivatives from radix Angelicae dahuricae and their effects on RXRα transcriptional regulation. Molecules 16:6339–6348
Franke K, Porzel A, Masaoud M, Adam G, Schmidt J (2001) Furanocoumarins from Dorstenia gigas. Phytochemistry 56:611–621
Fujioka T, Furumi K, Fujii H, Okabe H, Mihashi K, Nakano Y, Matsunaga H, Katano M, Mori M (1999) Antiproliferative constituents from umbelliferae plants. V. A new furanocoumarin and falcarindiol furanocoumarin ethers from the root of Angelica japonica. Chem Pharm Bull 47:96–100
Mendez J, Poceiro JC (1982) Furocoumarins from Angelica pachycarpa. Phytochemistry 22:2599–2601
Thanh PN, Jin WY, Dong GY, Bae K, Kang SS (2004) Cytotoxic coumarins from the root of Angelica dahurica. Arch Pharm Res 27:1211–1215
Seo WD, Kim JY, Ryu HW, Kim JH, Han SI, Ra JE, Seo KH, Jang KC, Lee JH (2013) Identification and characterisation of coumarins from the roots of Angelica dahurica and their inhibitory effects against cholinesterase. J Funct Foods 5:1421–1431
Lv X, Liu D, Hou J, Dong P, Zhan L, Wang L, Deng S, Wang C, Yao J, Shu X, Liu L, Ma X (2013) Biotransformation of imperatorin by Penicillium janthinellum. Anti-osteoporosis activities of its metabolites. Food Chem 138:2260–2266
Row EC, Brown SA, Stachulski AV, Lennard MS (2006) Synthesis of 8-geranyloxypsoralen analogues and their evaluation as inhibitors of CYP3A4. Bioorg Med Chem 14:3865–3871
Takasugi M, Anetai M, Katsui N, Masamune T (1973) The occurrence of vomifoliol, dehydrovomifoliol, and dihydrophaseic acid in the roots of “kidney bean”. Chem Lett 3:245–248
Sugimoto T, Ueno A, Kadota S, Cui C, Kikuchi T (1988) New 5β-H limonoids from Evodia rutaecarpa Bentham. Chem Pharm Bull 36:1237–1240
Liu J (2001) Two new limonoids from Polygonum orientaleI L. Indian J Chem 40B:644–646
Ziegler H, Spiteller G (1992) Coumarins and psoralens from Sicilian lemon oil (Citrus limon (L.) Burm. F.). Flavor Frag J 7:129–139
Olguin-Reyes S, Camacho-Carranza R, Hernandez-Ojeda S, Elinos-Baez M, Espinosa-Aguirre JJ (2012) Bergamottin is a competitive inhibitor of CYP1A1 and is antimutagenic in the Ames test. Food Chem Toxicol 50:3094–3099
Chevereau M, Glatti H, Zalko D, Cravedi JP, Audebert M (2017) Role of human sulfotransferase 1A1 and N-acetyltransferase 2 in the metabolic activation of 16 heterocyclic amines and related heterocyclics to genotoxicants in recombinant V79 cells. Arch Toxicol. doi:10.1007/s00204-017-1935-8
Celic A, Eke D, Ekinci YS, Yildirim S (2013) The protective role of curcumin on perfluorooctane sulfonate-induced genotoxicity: single cell gel electrophoresis and micronucleus test. Food Chem Toxicol 53:249–255
Acknowledgements
This research was supported by MEXT (Ministry of Education, Culture, Sports, Scientific and Technology)—Supported Program for the Strategic Research Foundation at Private Universities, 2015–2019 and by JSPS KAKENHI Grant Number JP17K15473.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsumoto, T., Takahashi, K., Kanayama, S. et al. Structures of antimutagenic constituents in the peels of Citrus limon . J Nat Med 71, 735–744 (2017). https://doi.org/10.1007/s11418-017-1108-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-017-1108-3