Abstract
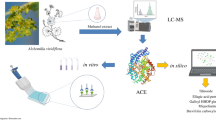
Members of the genus Limonium are widely used as medicinal herbs due to their health-promoting effects, such as an ability to improve blood circulation by inhibiting angiotensin I converting enzyme (ACE). While the potential of L. michelsonii Lincz. (a medicinal plant endemic to Kazakhstan) to inhibit ACE has been demonstrated, the inhibitory activities of its secondary metabolites have not been explored. In this work, the principal phenolic compounds (1–20) among these metabolites were isolated to determine the components responsible for ACE inhibition. The natural abundances of the active constituents within the target plant were characterized by UPLC-Q-TOF/MS analysis. All of the isolated compounds except for gallates 10–12 were found to significantly inhibit ACE, with IC50 values of between 7.1 and 138.4 μM. Unexpectedly, the flavonol glycosides 16–20 were observed to be more potent than the corresponding aglycones 4 and 5. For example, quercetin (4) had IC50 = 30.3 μM, whereas its glycosides (16, 17) had IC50 = 10.2 and 14.5 μM, respectively. A similar trend was observed for myricetin (5) and its glycosides (18–20). In a kinetic study, the flavonols 3–5 and 16–20 and the dihydroflavonols 8 and 9 behaved as competitive inhibitors, whereas other flavones (1, 2, 13–15) and flavanones (6, 7) performed noncompetitive inhibition.





Similar content being viewed by others
Abbreviations
- ACE:
-
Angiotensin I converting enzyme
- IC50 :
-
Inhibitor concentration that produces a 50% decrease in activity
- K i :
-
Inhibition constant
- V max :
-
Maximum velocity
- K m :
-
Michaelis–Menten constant
References
Baytenov MC (1963) Flora of Kazakhstan, part 7. Science Press, Almaty, p 75
Compiling Groups of Countrywide Herbal Medicine of China (1996) Compilation of countrywide herbal medicine of China, part 1, 2nd edn. People’s Medical Publishing House, Beijing, pp 407–408
Xu X, Bahargul K, Hang B, Jia XG (2009) Kazakh herbal medicine, part 1. The Ethnic Press, Beijing, pp 90–92
Aniya Y, Miyagi C, Nakandakari A, Kamiya S, Imaizumi N, Ichiba T (2002) Free radical scavenging action of the medicinal herb Limonium wrightii from the Okinawa Islands. Phytomed 9:239–244
Sihem B, Nicola M, Teresa M, Tiziana E, Rita PA, Noureddine B, Samir B, Massimiliano DA, Antonio V (2015) Phenolic compounds from Limonium pruinosum. Nat Pro Com 10:319–321
Anastassiya VG, Amer HT, Galiya EZh, Nadezhda GG, Charles LC, Stephen JC, Samir AR (2015) Sulfated phenolic compounds from Limonium caspium: isolation, structural elucidation, and biological evaluation. Fitoterapia 104:80–85
Tang XH, Yu F, Liu J, Gao J, Yan LF, Dong MM (2014) Isolation and identification of anti-tumor polysaccharide LSP21from Limonium sinense (Girard) Kuntze. Int J Biol Macromol 70:138–142
Kazuyoshi K, Mami T, Kotaro M, Yoshishisa T (2005) A novel drime-type sesquiterpene from Limonium wrightii. J Nat Med 59(4):186–188
Faten M, Wided M, Vakhtang M, Andre P, Jean L, St-G Alexis, Riadh K (2014) Antiviral-guided fractionation and isolation of phenolic compounds from Limonium densiflorum hydroalcoholic extract. C R Chimie 19:726–732
Medini F, Fellah H, Ksouri R, Abdelly C (2014) Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J Taibah Univ Sci 8(3):216–224
Kandil FE, Ahmed KM, Hussieny HA, Soliman AM (2000) A new flavonoid from Limonium axillare. Arch Pharm J Pharmacol Med Chem 333:275–277
Murray AP, Rodriguez S, Frontera MA, Tomas MA, Mulet MC (2004) Antioxidant metabolites from Limonium brasiliense (Boiss.) Kuntze. Z Naturforsch 59:477–480
Yuh ChK, Lie ChL, Wei JT, Cheng JC, Szu HK, Yen HH (2002) Samarangenin B from Limonium sinense suppresses herpes simplex virus type 1 replication in vero cells by regulation of viral macromolecular synthesis. Antimicrob Agents Chemother 46(9):2854–2864
Jallapally A, Addla D, Bagul P, Sridhar B, Banerjee SK, Kantevari S (2015) Design, synthesis and evaluation of novel 2-butyl-4-chloroimidazole derived peptidomimetics as angiotensin converting enzyme (ACE) inhibitors. Bioorg Med Chem 23:3526–3533
Shukor NA, Camp JV, Gonzales GB, Staljanssens D, Struijs K, Zotti MJ, Raes K, Smagghe G (2013) Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: a study of structure activity relationships. J Agric Food Chem 61:11832–11839
Felmeden DC, Lip GY (2000) The renin–angiotensin–aldosterone system and fibrinolysis. JRAAS 1(3):240–244
Mitsuo M, Masayoshi H (2003) Antimutagenic activity of flavonoids from Chrysanthemum morifolium. Biosci Biotechnol Biochem 67:2091–2099
Masuda T, Iritani K, Yonemori S, Oyama Y, Takeda Y (2001) Isolation and antioxidant activity of galloylflavonol glycosides from the seashore plant, Pemphis acidula. Biosc Biotechnol Biochem 65:1302–1309
Movsumov IS (1996) Flavonoids of the roots of Limonium caspium. Chem Nat Compd 32(6):922
Zhang XF, Hung TM, Phuong TP, Ngoc TM, Min BS, Song KS, Seong YH, Bae KH (2006) Anti-inflammatory activity of flavonoids from Populus davidiana. Arch Pharm Res 29(12):1102–1108
Jeon SH, Chun W, Choi YJ, Kwon YS (2008) Cytotoxic constituents from the bark of Salix hulteni. Arch Pharm Res 31(8):978–982
Trabelsi N, Oueslati S, Ksouri R, Nassra M, Marchal A, Krisa S, Abdelly C, Scharbert S, Holzmann N, Hofmann T (2004) Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse. J Agric Food Chem 52:3498–3508
Guvenalp Z, Ozbek H, Kuruuzum-uz A, Kazaz C, Demirezer LO (2009) Secondary metabolites from Nepeta heliotropifolia. Turk J Chem 33:667–675
Lin YL, Wang ChN, Shiao YJ, Liu TY, Wang WY (2003) Benzolignanoid and polyphenols from Origanum vulgare. J Chin Chem Soc 50:1079–1083
Hilbert G, Temsamani H, Bordenave L, Pedrot E, Chaher N, Cluzet S, Delaunay JC, Ollat N, Delrot S, Merillon JM, Gomes E, Richard T (2015) Flavonol profiles in berries of wild Vitis accessions using liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance spectrometry. Food Chem 169:49–58
Dawidar AM, Abdel-Mogib M, El-Nagga ME, Mostafa ME (2014) Isolation and characterization of Polygonum equisetiforme flavonoids and their acaricidal activity against Tetranychus urticae Koch. Res J Pharm Biol Chem Sci 5(4):140–148
Korul′kina LM, Shul′ts EE, Zhusupova GE, Abilov ZhA, Erzhanov KB, Chaudri MI (2014) Biologically active compounds from Limonium gmelinii and L. popovii. I. Chem Nat Compd 40(5):465–471
Sentandreu MA, Toldrá FA (2006) A fluorescence-based protocol for quantifying angiotensin-converting enzyme activity. Nat Protoc 1(5):2423–2437
Acknowledgements
This work was done with research funds from the Ministry of Agriculture, Food and Rural Affairs (No. 315032-04-2-SB010) and the Next-Generation BioGreen 21 program, Rural Development Administration (SSAC, No. PJ01107001), Republic of Korea. The BK21 PLUS program supported scholarships for senior researchers and all other students.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jenis, J., Kim, J.Y., Uddin, Z. et al. Phytochemical profile and angiotensin I converting enzyme (ACE) inhibitory activity of Limonium michelsonii Lincz. J Nat Med 71, 650–658 (2017). https://doi.org/10.1007/s11418-017-1095-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-017-1095-4