Abstract
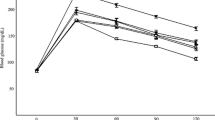
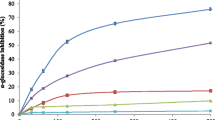
Seeds of Securigera securidaca (Fabaceae) are used in Iranian folk medicine as an antidiabetic treatment. In this study, the antihyperglycemic activity of chloroform and methanol fractions (CF and MF) from S. securidaca seed extract was investigated and their bioactive constituents were identified. The antidiabetic effects of fractions were assessed by streptozocin-induced diabetic Naval Medical Research Institute mice. The hypoglycemic activity of MF at 100 mg/kg and CF at 400 mg/kg was comparable with glibenclamide (3 mg/kg). MF at 400 mg/kg and CF at 600 mg/kg showed equal hypoglycemic responses to 12.5 IU/kg insulin (P > 0.05). Three cardiac glycosides were isolated as active constituents responsible for the hypoglycemic activity. Securigenin-3- O -β-glucopyranosyl-(1 → 4)-β-xylopyranoside (1) was a major compound in seeds. Securigenin-3- O -inositol-(1 → 3)-β-glucopyranosyl-(1 → 4)-β-xylopyranoside (2) and securigenin-3- O -α-rhamnopyranosyl-(1 → 4)-α-glucopyranoside (3) were found as new natural products. When 1–3 were tested at 10 mg/kg there was a significant reduction of blood glucose levels in diabetic mice, comparable to that of 3 mg/kg glibenclamide (P > 0.05). The hypoglycemic effect was due to an increase in insulin secretion; the insulin levels in the diabetic mice significantly improved and were comparable with those in healthy animals (P > 0.05). Compounds responsible for the hypoglycemic properties of S. securidaca seeds were identified as cardiac glycosides and were found to act via an increase of insulin levels in a diabetic mouse model.




Similar content being viewed by others
References
Rechinger KH (1984) Papilionaceae II. In: Rechinger KH (ed) Flora Iranica 157. Akademische Druck und Verlagsanstalt, Austria
Ali AA, Mohamed MH, Kamel MS, Fouad MA, Spring O (1998) Studies on Securigera securidaca (L.) Deg. Et Dorfl. (Fabaceae) seeds, an antidiabetic Egyptian folk medicine. Pharmazie 53:710–715
Porchezhian E, Ansari SH (2001) Effect of Securigera securidaca on blood glucose levels of normal and alloxan-induced diabetic rats. Pharm Biol 39:62–64
Nagarajan S, Jain HC, Aulakh GS (1982) Indigenous plants used in the control of diabetes. In: Atal C, Kapur B (eds) Cultivation and utilization of medicinal plants. Regional Research Laboratory, India, pp 584–604
Hosseinzadeh H, Ramezani M, Danaei AR (2002) Antihyperglycaemic effect and acute toxicity of Securigera securidaca L. seed extracts in mice. Phytother Res 16:745–747
Zahedi Asl S, Marahel H, Zare B (2005) Study on the effects of chloroformic extract of Securigera securidaca on serum glucose level and liver glycogen content of mice. J Kerman Univ Med Sci 12:32–38
Ghitasi I, Nikbakht MR, Sadeghi H, Sabzali V, Sabzali S, Shahrani M (2007) The hypoglycemic effects of a hydro-alcoholic extract from Securigera securidaca seeds on induced diabetic in male rats. J Shahrekord Univ Med Sci 8:68–73
Pouramir M, Shahaboddin ME, Moghadamnia AA, Parastouei K (2011) To study the effects of Securigera securidaca (L.) seed against alloxan-induced hyperglycemia. J Med Plants Res 5:3188–3191
Roostazadeh A, Firoozrai M, Shabani M (2008) Effect of aqueous seed extract of Securigera securidaca on erythrocytes catalase activity in type 1 diabetic rats. Qom Univ Med Sci J 1:9–14
Al-Hachhim G, Maki B (1969) Effect of Securigera securidaca on electroshock seizure threshold in mice. Psychol Rep 24:551–553
Mard SA, Bahari Z, Eshaghi N, Farbood Y (2008) Antiulcerogenic effect of Securigera securidaca L. seed extract on various experimental gastric ulcer models in rats. Pak J Biol Sci 11:2619–2623
Azarmiy Y, Zakheri A, Allaf Akbari N, Fathi Azad F, Fakhrjo A, Andalib S, Maleki Dizaji N, Babaei H, Garjani A (2009) The effect of total extract of Securigera securidaca L. seed on thoracic aorta function in nigh-fat fed rats. Pharm Sci 15:83–92
Garjani A, Fathiazad F, Zakheri A, Akbari NA, Azarmie Y, Fakhrjoo A, Andalib S, Maleki Dizaji N (2009) The effect of total extract of Securigera securidaca L. seeds on serum lipid profiles, antioxidant status, and vascular function in hypercholesterolemic rats. J Ethnopharmacol 126:525–532
Shahidi S, Pahlevani P (2013) Antinociceptive effects of an extract of Securigera securidaca and their mechanisms in mice. Neurophysiology 45:34–38
Tofighi Z, Asgharian P, Goodarzi S, Hadjiakhoondi A, Ostad SN, Yassa N (2014) Potent cytotoxic flavonoids from Iranian Securigera securidaca. Med Chem Res 23:1718–1724
Zamula VV, Maksyutina NP, Kolesnikov DG (1965) Cardenolides of Securigera securidaca II. Chem Nat Compd 1:117–119
Zatula VV, Chernobrovaya NV, Kolesnikov DG (1966) The structure of securigenin and its biosidesecuridaside. Khim Prir Soedin 2:438–439
Komissarenko AN, Kovalev VN (1987) Hydroxycoumarins and flavones of Securigera securidaca. Khim Prir Soedin 2:298–299
Tofighi Z, Alipour F, Hadavinia H, Abdollahi M, Hadjiakhoondi A, Yassa N (2014) Effective antidiabetic and antioxidant fractions of Otostegia persica extract and their constituents. Pharma Biol 52:961–966
Yassa N, Saeidnia S, Pirouzi R, Akbaripour M, Shafiee A (2007) Three phenolic glycosides and immunological properties of Achillea millefolium from Iran, population Golestan. Daru 15:49–52
Bruker (2006) Bruker Analytical X-Ray Systems, Inc. apex 2, version 2 ed. Madison, M86-E01078
Altomare A, Cascarano G, Giacovazzo C, Guagliardi A, Burla MC, Polidori G, Camalli M (1994) A program for automatic solution of crystal structures by direct methods optimized for powder data. J Appl Crystallogr 27:435–436
Betteridge PW, Carruthers JR, Cooper RI, Prout K, Watkin DJ (2003) CRYSTALS version 12: software for guided crystal structure analysis. J Appl Crystallogr 36:1487
Prince E (1982) Mathematical techniques in crystallography and materials science. Springer, New York, pp 12–13 (Duoc Hoc 6)
Watkin DJ (1994) The control of difficult refinements. Acta Crystallogr A 50:411–437
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, Streek JV, Wood PA (2008) Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J Appl Crystallogr 41:466–470
Ripperger HH, Lindig C, Snatzke G (1998) Circular dichroism of cardenolides. J Prak Chem-Chem ZTG 340:476–478
Bagirov RB, Komissarenko NF (1966) New cardenolides from the seeds of Coronilla hyrcana. Khim Prir Soedin 2:251–257
Hembree JA, Chang CJ, McLaughlin JL, Peck G, Cassady JM (1979) Potential antitumor agents: a cytotoxic cardenolide from Corronilla varia. J Nat Prod 42:293–298
Ankli A, Heilmann J, Heinrich M, Sticher O (2000) Cytotoxic cardenolides and antibacterial terpenoids from Crossopetalum gaumeri. Phytochemistry 54:531–537
Kasai R, Okihara M, Asakawa J, Mizutani K, Tanaka O (1979) 13C NMR study of a- and b-anomeric pairs of d-mannopyranosides and l-rhamnopyranosides. Tetrahedron 35:1427–1432
Marles RJ, Farnsworth NR (1995) Antidiabetic plants and their active constituents. Phytomedicine 2:137–189
Ross IA (2001) Medicinal plants of the world-chemical constituents. In: Traditional and modern uses. Human Press Inc, Totowa
Mraz M, Opletal L, Sovova M, Drasar P, Havel M (1992) Inhibition of Na+–K+ATPase by the glycosides from Coronilla varia. Planta Med 58:467–468
Ahmad VU, Basha A (2006) Cardenolides and pregnanes. In: Ahamad VU, Basha A (ed) Spectroscopic data of steroid glycosides. Springer, New York, p 2335
Triner L, Killian P, Nahas GG (1968) Ouabain hypoglycemia: insulin mediation. Science 162:560–561
Triner L, Papayoanou J, Killian P, Vulliemoz Y, Castany R, Nahas GG (1969) Effects of ouabain on insulin secretion in the dog. Circ Res 25:119–129
Oubaassinea R, Weckering M, Kessler L, Breidert M, Roegel JC, Eftekhari P (2012) Insulin interacts directly with Na+/K+ATPase and protects from digoxin toxicity. Toxicology 299:1–9
Aubie A, Naranjan D, Grant P, Pawan S (2001) Diabetes and cardiovascular disease etiology, treatment and outcomes. Springer, NewYork
Acknowledgements
This research was a part of PhD thesis and it was supported by Tehran University of Medical Sciences and Health Services Grants (Nos. 11981 and 13726).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tofighi, Z., Moradi-Afrapoli, F., Ebrahimi, S.N. et al. Securigenin glycosides as hypoglycemic principles of Securigera securidaca seeds. J Nat Med 71, 272–280 (2017). https://doi.org/10.1007/s11418-016-1060-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-016-1060-7