Abstract
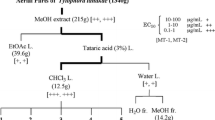
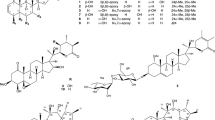
During the course of our studies towards the identification of promising chemotherapeutic candidates from plants against two human T-cell lymphotropic virus type I-infected T-cell lines (MT-1 and MT-2), we screened 17 extracts from 9 rutaceous plants against MT-1 and MT-2 cells. The extracts from the aerial parts and roots of Boenninghausenia japonica, as well as the leaves and roots of Ruta graveolens showed potent antiproliferative effects. After activity-guided fractionation, we isolated 44 compounds from two rutaceous plants, including three new compounds (1–3), which were classified into 26 coumarin analogs (13 coumarins, 8 furanocoumarins, 4 dihydrofuranocoumarins and one dihydropyranocoumarin), 15 alkaloid analogs (7 quinolone alkaloids, 4 acridone alkaloids, 3 furanoquinoline alkaloids and one tetrahydroacridone alkaloid) and 3 flavonoid glycosides. Structure–activity relationship studies were also evaluated. The coumarin compounds (2, 3 and 7–9) bearing a 3-dimethylallyl moiety showed potent activity. Similarly, of all the furanocoumarins evaluated in the current study, compound 17 bearing a 3-dimethylallyl group also showed potent activity. A dihydrofuranocoumarin (27) bearing a 3-dimethylallyl moiety showed the most potent activity. Following 27, compound 28 showed potent activity. These results therefore suggested that the presence of a 3-dimethylallyl moiety was important to the antiproliferative activity of these coumarin analogs.







Similar content being viewed by others
References
Ishitsuka K, Tamura K (2008) Treatment of adult T-cell leukemia/lymphoma: past, present, and future. Eur J Haematol 80:185–196
Nakano D, Ishitsuka K, Hatsuse T, Tsuchihashi R, Okawa M, Okabe H, Tamura K, Kinjo J (2011) Screening of promising chemotherapeutic candidates against human adult T-cell leukemia/lymphoma from plants: active principles structure–activity relationships with withanolides. J Nat Med 65:559–567
Nakano D, Ishitsuka K, Katsuya H, Kunami N, Nogami R, Yoshimura Y, Matsuda M, Kamikawa M, Tsuchihashi R, Okawa M, Ikeda T, Nohara T, Tamura K, Kinjo J (2013) Screening of promising chemotherapeutic candidates from plants against human adult T-cell leukemia/lymphoma (II): apoptosis of antiproliferactive principle (24,25-dihydrowithanolide D) against ATL cell lines and structure–activity relationships with withanolides isolated from solanaceous plants. J Nat Med 67:415–420
Nakano D, Ishitsuka K, Kamikawa M, Matsuda M, Tsuchihashi R, Okawa M, Okabe H, Tamura K, Kinjo J (2013) Screening of promising chemotherapeutic candidates from plants against human adult T-cell leukemia/lymphoma (III). J Nat Med 67:894–903
Nakano D, Ishitsuka K, Ikeda M, Tsuchihashi R, Okawa M, Okabe H, Tamura K, Kinjo J (2015) Screening of promising chemotherapeutic candidates from plants against human adult T-cell leukemia/lymphoma (IV): phenanthroindolizidine alkaloids from Tylophora tanakae leaves. J Nat Med 69:397–401
Harborne JB (1988) The flavonoids. Advances in research. Chapman and Hall, New York
Chaya N, Terauchi K, Yamagata Y, Kinjo J, Okabe H (2004) Antiproliferative constituents in plants 14. Alkaloids from Boenninghausenia japonica Nakai. Biol Pharm Bull 27:1312–1316
Dieter B, Kalman S, Johannes R (1977) 13C-NMR-Spektren einiger C-3 prenylierter Rutaceen-Cumarine. Arch Pharm 310:390–393
Dieter B, Zsuzsa R, Iuliu M, Johannes R (1978) Beitrag zur 13C-NMR-Spektroskopie von Rutaceen-Cumarinen. Arch Pharm 311:1026–1029
Shibata S, Noguchi M (1977) Two new coumarins in Boenninghausenia albiflora. Phytochemistry 16:291–293
John L, Marawan S (1984) Coumarin sulphates of Seseli libanotis. Phytochemistry 23:863–865
Joshi PC, Mandal S, Das PC, Chatterjee A (1993) Twominor coumarins of Boenninghausenia albiflora. Phytochemistry 32:481–483
Sudam CB, Durga PD, Rabindra NT (1984) Bhubaneswin a new blcoumarin. Heterocycle 22:333–337
Kinoshita T, Jin BW, Feng CH (1996) The isolation of a prenylcoumarin of chemotaxonomic significance from Murraya paniculata var. omphalocarpa. Phytochemistry 43:125–128
Kozawa M, Baba K, Minami M, Nitta H, Hata K (1974) Uber die cumarine der Boenninghausenia japonica (sieb.) Nakai. Chem Pharm Bull 22:2746–2749
Elgamal MHA, Elewa NH, Elkhrisy EAM, Duddeck H (1979) 13C NMR chemical shifts and carbon-proton coupling constants of some furocoumarins and furochromones. Phytochemistry 18:139–143
Adeleke CA, Johannes R (2000) Minor furocoumarins of Murraya koenigii. Fitoteraoia 71:334–337
Xue MN, Sheng HL, Li XW, Ling L, Li HG, Han DS (2004) Two new coumarin derivatives from the roots of Heracleum rapula. Lett Planta Med 70:578–581
Abyshev AZ, Agaev EM, Balabudkin MA (1993) Rutarin from the roots of Seseli grandivittatum. Chem Nat Compd 29:250–251
Fernando M, Antonio E, Lucia M, Jose G, Giselle M (1996) Alkaloids and coumarins from esenbeckia species. Phytochemistry 41:647–649
Purusotam B, Shigetoshi K, Krishna M, Mangala DM, Tsuneo N (1993) Constituents of Boenninghausenia albiflora: isolation and identification of some coumarins. Planta Med 59:384–386
Marumoto S, Miyazawa M (2011) Microbial reduction of coumarin, psoralen, and xanthyletin by Glomerella cingulata. Tetrahedron 67:495–500
Monira A, Alexander IG, Greg L, Peter GW (1993) Quinolone and acridone alkaloids from Boronia lanceolata. Phytochemistry 33:1507–1510
Bergenthal D, Mester I, Rozsa Z, Reisch J (1979) 13C-NMR-Spektren einiger acridon-alkaloide. Phytochemistry 18:161–163
Zs Rozsa, Szendrei K, Kovacs Z, Novak I, Minker E, Reisch J (1978) The co-occurrence of rutacridone and noracronycine in the roots of Boenninghausenia albiflora. Phytochemistry 17:169–170
Reisch J, Szendrei K, Minker E, Novak I (1969) Quinoline alkaloids from Ruta graveolens. XXIV N-methylplatydesminium and ribalindine. Pharmazie 24:699–700
Jagadeesh SG, David KGL, Srimannarayana G (2000) Antifeedant activity of the constituents of Evodia lunu-ankenda. Indian J Chem 39B:475–476
Narasimhan NS, Mali RS (1974) Synthetic application of lithiation reactions VI. Tetrahedron 30:4153–4157
Elaine MC, James AM, Marcelo RS, Luis OR, Simone YS, Norberto PL, Jose RP, Vanderlan SB, Maria CM (2010) Alkaloids from stems of Esenbeckia leiocarpa engl. (Rutaceae) as potential treatment for alzheimer disease. Molecules 15:9205–9213
Taniguchi M, Satomura Y (1972) Structure and physiological activity of carbostyril compounds. Agric Biol Chem 36:2169–2175
Yuan QT, Xiao ZF, Liang H (1996) Quinolone alkaloids from Evodia rutaecarpa. Phytochemistry 43:719–722
Annunziata R, Cenini S, Palmisano G, Tollari S (1996) 4(1H)-quinolinone alkaloids. An efficient synthesis of graveoline by palladium-catalysed reductive N-heterocyclisation. Synth Commun 26:495–501
Khalid ES, Mansour SA, Farouk SE, Samir AR (2000) New quinolone alkaloids from Ruta chalepensis. J Nat Prod 63:995–997
Finn GJ, Creaven BS, Egan DA (2004) A study of the role of cell cycle events mediating the mechanism of action of coumarin derivatives in human malignant melanoma cells. Cancer Lett 214:43–54
Finn GJ, Creaven BS, Egan DA (2004) Daphnetin-induced differentiation of human renal carcinoma cells and its mediation by p38 mitogen-activated protein kinases. Biochem Pharm 67:1779–1788
Finn GJ, Creaven BS, Egan DA (2005) Effects of coumarin derivatives on differentiation of melanotic malanoma cells: a functional role for mitogen-activated protein kinases. Eur J Pharm Sci 26:16–25
Klenkar J, Molnar M (2015) Natural and synthetic coumarins as potential anticancer agents. J Chem Pharm Res 7:1223–1238
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakano, D., Ishitsuka, K., Matsuda, N. et al. Screening of promising chemotherapeutic candidates from plants against human adult T-cell leukemia/lymphoma (V): coumarins and alkaloids from Boenninghausenia japonica and Ruta graveolens . J Nat Med 71, 170–180 (2017). https://doi.org/10.1007/s11418-016-1046-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-016-1046-5