Abstract
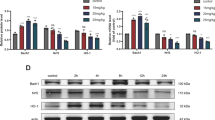
Glycyrrhetinic acid (GA) is an ingredient of triterpene saponins found in Gancao (Radix Glycyrrhizae). Here, we investigated the protective effects of GA in H9c2 cells, and explored its possible mechanism of action. Different concentrations of GA were used to treat H9c2 cells under oxygen glucose deprivation. We analyzed cell necrosis and apoptosis using optical microscopy, Hoechst 33342 staining, FITC-annexin V/PI double-staining and lactate dehydrogenase (LDH), creatine kinase-MB (CK-MB) and interleukin (IL)-1β assays. Changes in related pro-apoptosis and anti-apoptosis proteins were detected by Western blot. Optical microscopy showed that GA improved cell morphology, including cell shrinkage, cauliflower-like membrane blebbing, and even some cell debris. Meanwhile, GA also ameliorated cell nuclei characteristics such as nucleus size, chromatin condensation and bright staining from Hoechst 33342 staining. GA also lowered the apoptotic rate and the levels of LDH, CK-MB and IL-1β in a dose-dependent manner. Furthermore, GA treatment increased Bcl-2 protein expression and decreased caspase-8 and Bax protein expression, while elevating the Bcl-2/Bax ratio. GA preconditioning increased p-AKt protein expression; however, after adding LY 294002, the p-AKt expression decreased obviously. Our results demonstrated that GA could protect H9c2 cells from apoptosis in a dose-dependent manner, and the potential mechanism might be related to the PI3K/AKt signaling pathway.






Similar content being viewed by others
References
Forouzanfar MH, Moran AE, Flaxman AD, Roth G, Mensah GA, Ezzati M, Naghavi M, Murray CJ (2012) Assessing the global burden of ischemic heart disease, part 2: analytic methods and estimates of the global epidemiology of ischemic heart disease in 2010. Glob Heart 7:331–342
Heusch G (2013) Cardioprotection: chances and challenges of its translation to the clinic. Lancet 381:166–175
Tullio F, Angotti C, Perrelli MG, Penna C, Pagliaro P (2013) Redox balance and cardioprotection. Basic Res Cardiol 108:392
Marambio P, Toro B, Sanhueza C, Troncoso R, Parra V, Verdejo H, Garcia L, Quiroga C, Munafo D, Diaz-Elizondo J, Bravo R, Gonzalez MJ, Diaz-Araya G, Pedrozo Z, Chiong M, Colombo MI, Lavandero S (2010) Glucose deprivation causes oxidative stress and stimulates aggresome formation and autophagy in cultured cardiac myocytes. Biochim Biophys Acta 1802:509–518
Qiao S, Mao X, Wang Y, Lei S, Liu Y, Wang T, Wong GT, Cheung CW, Xia Z, Irwin MG (2016) Remifentanil preconditioning reduces postischemic myocardial infarction and improves left ventricular performance via activation of the janus activated kinase-2/signal transducers and activators of transcription-3 signal pathway and subsequent inhibition of glycogen synthase kinase-3beta in rats. Crit Care Med 44:e131–e145
Hung YC, Tseng YJ, Hu WL, Chen HJ, Li TC, Tsai PY, Chen HP, Huang MH, Su FY (2015) Demographic and prescribing patterns of Chinese herbal products for individualized therapy for ischemic heart disease in Taiwan: population-based study. PLoS One 10:e0137058
Peng F, Du Q, Peng C, Wang N, Tang H, Xie X, Shen J, Chen J (2015) A review: the pharmacology of isoliquiritigenin. Phytother Res 29:969–977
Kilgore KS, Tanhehco EJ, Park JL, Naylor KB, Anderson MB, Lucchesi BR (1998) Reduction of myocardial infarct size in vivo by carbohydrate-based glycomimetics. J Pharmacol Exp Ther 284:427–435
Wang L, Yang R, Yuan B, Liu Y, Liu C (2015) The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm Sin B 5:310–315
Zhang Q, Ye M (2009) Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J Chromatogr A 1216:1954–1969
Wu HJ, Yang JY, Jin M, Wang SQ, Wu DL, Liu YN, Yan X, Yang C, Zhang G, He J (2015) Glycyrrhetinic acid protects the heart from ischemia/reperfusion injury by attenuating the susceptibility and incidence of fatal ventricular arrhythmia during the reperfusion period in the rat hearts. Cell Physiol Biochem 36:741–752
Li X, Wu Y, Guo Z, Liu L (1992) Effect of sodium 18 beta-glycyrrhetate on experimental arrhythmia. Zhongguo Zhong Yao Za Zhi 17:176–178 (inside backcover)
Fan R, Li N, Xu H, Xiang J, Wang L, Gao Y (2016) The mechanism of hydrothermal hydrolysis for glycyrrhizic acid into glycyrrhetinic acid and glycyrrhetinic acid 3-o-mono-beta-d-glucuronide in subcritical water. Food Chem 190:912–921
Feng X, Ding L, Qiu F (2015) Potential drug interactions associated with glycyrrhizin and glycyrrhetinic acid. Drug Metab Rev 47:229–238
Rizvi M, Jawad N, Li Y, Vizcaychipi MP, Maze M, Ma D (2010) Effect of noble gases on oxygen and glucose deprived injury in human tubular kidney cells. Exp Biol Med (Maywood) 235:886–891
Zhou TMYJ, Wan HT, Zhang YY, Zhou HF (2014) Protective effect of main component combinations of Fuzi (Radix Aconiti Lateralis praeparata) and Gancao (Radix Glycyrrhizae) on passage myocardial cells injured by aconitine in rats. J Beijing Univ Tradit Chin Med 37:22–27 (in Chinese)
Liao ZPLD, Wang SX, Li WJ, Chen HP, He M (2012) Protective effect of AE3 on anoxia preconditioning via NO pathway in rat cardiomyocytes. Chin Pharmacol Bull 28:39–43 (in Chinese)
Hemmings BA, Restuccia DF (2012) PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol 4:1485–1495
Gordon JW, Shaw JA, Kirshenbaum LA (2011) Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res 108:1122–1132
Yan X, Yang X, Hao X, Ren Q, Gao J, Wang Y, Chang N, Qiu Y, Song G (2015) Sodium fluoride induces apoptosis in H9c2 cardiomyocytes by altering mitochondrial membrane potential and intracellular ROS level. Biol Trace Elem Res 166:210–215
Zhang H, Xiong Z, Wang J, Zhang S, Lei L, Yang L, Zhang Z (2016) Glucagon-like peptide-1 protects cardiomyocytes from advanced oxidation protein product-induced apoptosis via the PI3K/Akt/Bad signaling pathway. Mol Med Rep 13:1593–1601
Festjens N, van Gurp M, van Loo G, Saelens X, Vandenabeele P (2004) Bcl-2 family members as sentinels of cellular integrity and role of mitochondrial intermembrane space proteins in apoptotic cell death. Acta Haematol 111:7–27
van Empel VP, Bertrand AT, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ (2005) Myocyte apoptosis in heart failure. Cardiovasc Res 67:21–29
Mughal W, Dhingra R, Kirshenbaum LA (2012) Striking a balance: autophagy, apoptosis, and necrosis in a normal and failing heart. Curr Hypertens Rep 14:540–547
Qanungo S, Das M, Haldar S, Basu A (2005) Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and Caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis 26:958–967
Haunstetter A, Izumo S (1998) Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res 82:1111–1129
MacLellan WR, Schneider MD (1997) Death by design. Programmed cell death in cardiovascular biology and disease. Circ Res 81:137–144
Dorn GW 2nd, Force T (2005) Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest 115:527–537
Papanicolaou KN, Izumiya Y, Walsh K (2008) Forkhead transcription factors and cardiovascular biology. Circ Res 102:16–31
Shioda N, Ishigami T, Han F, Moriguchi S, Shibuya M, Iwabuchi Y, Fukunaga K (2007) Activation of phosphatidylinositol 3-kinase/protein kinase B pathway by a vanadyl compound mediates its neuroprotective effect in mouse brain ischemia. Neuroscience 148:221–229
Hu X, Xu C, Zhou X, Cui B, Lu Z, Jiang H (2011) PI3K/Akt signaling pathway involved in cardioprotection of preconditioning with high mobility group box 1 protein during myocardial ischemia and reperfusion. Int J Cardiol 150:222–223
Theberge JF, Mehdi MZ, Pandey SK, Srivastava AK (2003) Prolongation of insulin-induced activation of mitogen-activated protein kinases ERK 1/2 and phosphatidylinositol 3-kinase by vanadyl sulfate, a protein tyrosine phosphatase inhibitor. Arch Biochem Biophys 420:9–17
Jiang E, Sun X, Kang H, Sun L, An W, Yao Y, Hu X (2015) Dehydrocostus lactone inhibits proliferation, antiapoptosis, and invasion of cervical cancer cells through PI3K/Akt signaling pathway. Int J Gynecol Cancer 25:1179–1186
Zou Y, Wang B, Fu W, Zhou S, Nie Y, Tian S (2016) Apelin-13 protects PC12 cells from corticosterone-induced apoptosis through PI3K and ERKs activation. Neurochem Res 41:1635–1644
Matsui T, Rosenzweig A (2005) Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol 38:63–71
Han D, Huang W, Ma S, Chen J, Gao L, Liu T, Zhang R, Li X, Li C, Fan M, Chen Y, Cao F (2015) Ghrelin improves functional survival of engrafted adipose-derived mesenchymal stem cells in ischemic heart through PI3K/Akt signaling pathway. Biomed Res Int. doi:10.1155/2015/858349
Wang J, Ji SY, Liu SZ, Jing R, Lou WJ (2015) Cardioprotective effect of breviscapine: inhibition of apoptosis in H9c2 cardiomyocytes via the PI3K/Akt/eNOS pathway following simulated ischemia/reperfusion injury. Pharmazie 70:593–597
Zhang J, He Z, Guo J, Li Z, Wang X, Yang C, Cui X (2016) Sulfiredoxin-1 protects against simulated ischaemia/reperfusion injury in cardiomyocyte by inhibiting PI3K/AKT-regulated mitochondrial apoptotic pathways. Biosci Rep. doi:10.1042/BSR20160076
Acknowledgments
This study is supported by grants from the National Nature Science Foundation of China (No. 81473412); Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents; Zhejiang Provincial Chinese Medicine (Combine Traditional Chinese and Western Medicine) Key Subject (No. 2012-XK-A06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no potential conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Wang, L., Zhang, Y., Wan, H. et al. Glycyrrhetinic acid protects H9c2 cells from oxygen glucose deprivation-induced injury through the PI3K/AKt signaling pathway . J Nat Med 71, 27–35 (2017). https://doi.org/10.1007/s11418-016-1023-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-016-1023-z