Abstract
Purpose
The subjects of this study were to investigate the remediating potential of the co-cultivation of Pleurotus eryngii and Coprinus comatus on soil that is co-contaminated with heavy metal (cadmium (Cd)) and organic pollutant (endosulfan), and the effects of the co-cultivated mushrooms on soil biochemical indicators, such as laccase enzyme activity and bacterial counts.
Materials and methods
A pot experiment was conducted to investigate the combined bioremediation effects on co-contaminated soil. After the mature fruiting bodies were harvested from each pot, the biomass of mushrooms was recorded. In addition, bacterial counts and laccase enzyme activity in soil were determined. The content of Cd in mushrooms and soil was detected by the flame atomic absorption spectrometry (FAAS), and the variations of Cd fractions in soil were determined following the modified BCR sequential extraction procedure. Besides, the residual endosulfan in soil was detected by gas chromatography-mass spectrometry (GC-MS).
Results and discussion
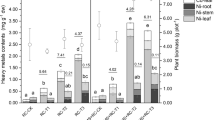
The results indicated that co-cultivation of P. eryngii and C. comatus exerted the best remediation effect on the co-contaminated soil. The biomass of mushroom in the co-cultivated group (T group) was 1.57–13.20 and 19.75–56.64% higher than the group individually cultivated with P. eryngii (P group) or C. comatus (C group), respectively. The concentrations of Cd in the fruiting bodies of mushrooms were 1.83–3.06, 1.04–2.28, and 0.67–2.60 mg/kg in T, P, and C groups, respectively. Besides, the removal rates of endosulfan in all treatments exceeded 87%. The best bioremediation effect in T group might be caused by the mutual promotion of these two kinds of mushrooms.
Conclusions
The biomass of mushroom, laccase activity, bacterial counts, and Cd content in mushrooms were significantly enhanced, and the dissipation effect of endosulfan was slightly higher in the co-cultivated group than in the individually cultivated groups. In this study, the effect of co-cultivated macro fungi P. eryngii and C. comatus on the remediation of Cd and endosulfan co-contaminated soil was firstly reported, and the results are important for a better understanding of the co-remediation for co-contaminated soil.







Similar content being viewed by others
References
Akpinar M, Urek RO (2014) Extracellular ligninolytic enzymes production by Pleurotus eryngii on agroindustrial wastes. Prep Biochem Biotechol 44:772–781
Bai Z, Harvey LM, Mcneil B (2003) Oxidative stress in submerged cultures of fungi. Crit Rev Biotechnol 23:267–302
Baldrian P (2003) Interactions of heavy metals with white-rot fungi. Enzym Microb Techol 32:78–91
Baldrian P (2008) Wood-inhabiting ligninolytic basidiomycetes in soils: ecology and constraints for applicability in bioremediation. Fungal Ecol 1:4–12
Baldrian P, Wiesche CID (2000) Influence of cadmium and mercury on activities of ligninolytic enzymes and degradation of polycyclic aromatic hydrocarbons by Pleurotus ostreatus in soil. Appl Environ Microbiol 66:2471–2478
Batty LC, Anslow M (2008) Effect of a polycyclic aromatic hydrocarbon on the phytoremediation of zinc by two plant species (Brassica juncea and Festuca arundinacea). Int J Phytoremediation 10:236–251
Cen F, Chen L, Hu Y, Xu H (2011) Chelator-induced bioextraction of heavy metals from artificially contaminated soil by mushroom (Coprinus comatus). Chem Ecol 28:1–14
Cen F, Hu Y, Xu H (2012) Responses of antioxidant defenses in Coprinus comatus exposed to cadmium and mercury toxicity. Asian J Chem 24:4679–4685
Chaiyama V, Petcharat V, Kritsaneepaiboon P (2007) Some morphological and physiological aspects and cultivation of Coprinus comatus (O. F. Mull.) Gray Songklanakarin. J Sci Technol 29:261–274
Chavez E, He ZL, Stoffella PJ, Mylavarapu R, Li Y, Baligar VC (2016) Evaluation of soil amendments as a remediation alternative for cadmium-contaminated soils under cacao plantations. Environ Sci Pollut Res 23:1–10
Chen GQ, Yun C, Zeng GM, Zhang JC, Chen YN, Liang W, Zhang WJ (2010) Speciation of cadmium and changes in bacterial communities in red soil following application of cadmium-polluted compost. Environ Eng Sci 27:2169–2192
Chigbo C, Batty L, Bartlett R (2013) Interactions of copper and pyrene on phytoremediation potential of Brassica juncea in copper–pyrene co-contaminated soil. Chemosphere 90:2542–2548
Forootanfar H, Movahednia MM, Yaghmaei S, Tabatabaei-Sameni M, Rastegar H, Sadighi A, Faramarzi MA (2012) Removal of chlorophenolic derivatives by soil isolated ascomycete of Paraconiothyrium variabile and studying the role of its extracellular laccase. J Hazard Mater 209-210:199–203
Gadd GM (1994) Interactions of fungi with toxic metals. Springer, US
Gogolev A, Wilke BM (1997) Combination effects of heavy metals and fluoranthene on soil bacteria. Biol Fertil Soils 25:274–278
Hadibarata T, Kristanti RA, Hamdzah M (2014) Biosorption and biotransformation of fluoranthene by the white-rot fungus Pleurotus eryngii F032. Biotechnol Appl Biochem 61:377–388
Huang H et al (2011) The phytoremediation potential of bioenergy crop Ricinus communis for DDTs and cadmium co-contaminated soil. Bioresour Technol 102:11034–11038
Hussain S, Arshad M, Saleem M, Khalid A (2008) Biodegradation of alpha- and beta-endosulfan by soil bacteria. Biodegradation 18:731–740
Jiang J, Liu H, Qiao L, Ni G, Yuan Y, Xu H (2015a) Combined remediation of Cd–phenanthrene co-contaminated soil by Pleurotus cornucopiae and Bacillus thuringiensis FQ1 and the antioxidant responses in Pleurotus cornucopiae. Ecotoxicol Environ Saf 120:386–393
Jiang J, Qin C, Shu X, Rong C, Song H, Qiao L, Xu H (2015b) Effects of copper on induction of thiol-compounds and antioxidant enzymes by the fruiting body of Oudemansiella radicata. Ecotoxicol Environ Saf 111:60–65
Kamei I, Takagi K, Kondo R (2011) Degradation of endosulfan and endosulfan sulfate by white-rot fungus Trametes hirsuta. J Wood Sci 57:317–322
Kang MS et al (2000) Studies on mycelial growth and artificial cultivation of Pleurotus eryngii. Korean J Mycol 28
Kathpal TS, Singh A, Dhankhar JS, Singh G (1997) Fate of endosulfan in cotton soil under sub-tropical conditions of northern India. Pestic Sci 50:21–27
Krastanov A (2000) Removal of phenols from mixtures by co-immobilized laccase/tyrosinase and polyclar adsorption. J Ind Microbiol Biotechnol 24:383–388
Krishnamurti GSR, Naidu R (2000) Speciation and phytoavailability of cadmium in selected surface soils of South Australia. Soil Res 38:991–1004
Li YJ, Hu PJ, Zhao J, Dong CX (2015) Remediation of cadmium- and lead-contaminated agricultural soil by composite washing with chlorides and citric acid. Environ Sci Pollut Res 22:5563–5571
Liu H, Guo S, Kai J, Hou J, Han X, Xu H (2015) Bioremediation of soils co-contaminated with heavy metals and 2,4,5-trichlorophenol by fruiting body of Clitocybe maxima. J Hazard Mater 294:121–127
Lu M, Xu K, Chen J (2013) Effect of pyrene and cadmium on microbial activity and community structure in soil. Chemosphere 91:491–497
Lu M, Zhang ZZ, Wang JX, Zhang M, Xu YX, Wu XJ (2014) Interaction of heavy metals and pyrene on their fates in soil and tall fescue (Festuca arundinacea). Environ Sci Technol 48:1158–1165
Martino E, Franco B, Piccoli G, Stocchi V, Perotto S (2002) Influence of zinc ions on protein secretion in a heavy metal tolerant strain of the ericoid mycorrhizal fungus Oidiodendron maius. Mol Cell Biochem 231:179–185
Najafi S, Jalali M (2015) Effects of organic acids on cadmium and copper sorption and desorption by two calcareous soils. Environ Monit Assess 187:1–10
Narin I, Soylak M, Dogan M (1997) Traffic pollution in Nigde-Turkiye: investigation of trace element contents of soil samples. Fresenius Environ Bull 6:749–752
Perronnet K, Schwartz C, Gérard E, Morel JL (2000) Availability of cadmium and zinc accumulated in the leaves of Thlaspi caerulescens incorporated into soil. Plant Soil 227:257–263
Qi L, Wang Z, Song M, Chen Y (2006) Evaluation of dissipation mechanisms by Lolium perenne L, and Raphanus sativus for pentachlorophenol (PCP) in copper co-contaminated soil. Sci Total Environ 368:814–822
Ribeiro B et al (2008) Comparative study of phytochemicals and antioxidant potential of wild edible mushroom caps and stipes. Food Chem 110:47–56
Rivero A, Niell S, Cesio V, Cerdeiras MP, Heinzen H (2012) Analytical methodology for the study of endosulfan bioremediation under controlled conditions with white rot fungi. J Chromatogr B 907:168–172
Roy P, Dhara K, Manassero M, Banerjee P (2000) Effects of long-term contamination of DDT on soil microflora with special reference to soil algae and algal transformation of DDT. Environ Pollut 109:35–42
Sikkema J, de Bont JA, Poolman B (1994) Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem 269:8022–8028
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404
Souza SCRD, Andrade SALD, Souza LAD, Schiavinato MA (2012) Lead tolerance and phytoremediation potential of Brazilian leguminous tree species at the seedling stage. J Environ Manag 110:299–307
Soylak M, Türkoglu O (1999) Trace metal accumulation caused by traffic in an agricultural soil near a motorway in Kayseri, Turkey. J Trace Microprobe Tech 17:209–217
Sun Y, Zhou Q, Xu Y, Wang L, Liang X (2011) Phytoremediation for co-contaminated soils of benzo[a]pyrene (B[a]P) and heavy metals using ornamental plant Tagetes patula. J Hazard Mater 186:2075–2082
Sungur A, Soylak M, Yilmaz E (2015) Characterization of heavy metal fractions in agricultural soils by sequential extraction procedure: the relationship between soil properties and heavy metal fractions. Soil Sediment Contam 24:1–15
Tang X, Dong S, Shi W, Gao N, Zuo L, Xu H (2016) Fates of nickel and fluoranthene during the bioremediation by Pleurotus eryngii in three different soils. J Basic Microbiol 56:1194–1202
Ulčnik A, Cigić IK, Pohleven F (2013) Degradation of lindane and endosulfan by fungi, fungal and bacterial laccases. World J Microbiol Biotechnol 29:2239–2247
Vig K, Megharaj M, Sethunathan N, Naidu R (2003) Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Adv Environ Res 8:121–135
Wang C, Yu D, Shi W, Jiao K, Wu B, Xu H (2016) Application of spent mushroom (Lentinula edodes) substrate and acclimated sewage sludge on the bioremediation of polycyclic aromatic hydrocarbon polluted soil. RSC Adv 6:37274–37285
Wang K, Zhu Z, Huang H, Li T, He Z, Yang X, Alva A (2012) Interactive effects of Cd and PAHs on contaminants removal from co-contaminated soil planted with hyperaccumulator plant Sedum alfredii. J Soils Sediments 12:556–564
Wang S, Mulligan CN (2004) Rhamnolipid foam enhanced remediation of cadmium and nickel contaminated soil. Water Air and Soil Pollut 157:315–330
Wu B (2015) Mycoremediation potential of Coprinus comatus in soil co-contaminated with copper and naphthalene. RSC Adv 5:67524–67531
Xiang L, Ding S (2010) Effect of Cu 2+, Mn 2+ and aromatic compounds on the production of laccase isoforms by Coprinus comatus. Mycoscience 51:68–74
Xu SY, Chen YX, Wu WX, Wang KX, Lin Q, Liang XQ (2006) Enhanced dissipation of phenanthrene and pyrene in spiked soils by combined plants cultivation. Sci Total Environ 363:206–215
Ying L, Feng Z, Jie C, Gan H, Guo Y (2007) Chemical fractionation of heavy metals in urban soils of Guangzhou. China Environ Monit Asses 134:429–439
Yoshida H, Sugahara T, Hayashi J (1986) Studies on free sugars, free sugaralcohols and organic acids of wild mushrooms. J Jpn Soc Food Sci 33:426–433
Zhang GQ, Wang YF, Zhang XQ, Ng TB, Wang HX (2010) Purification and characterization of a novel laccase from the edible mushroom Clitocybe maxima. Process Biochem 45:627–633
Zhang RF, Cui ZL, Jian HE, Huang TT, Shun-Peng LI (2004) Ecological effects of long-term methylparathion contamination on soil microflora. Rural Eco-environ 20:48–50
Zhu MJ, Du F, Zhang GQ, Wang HX, Ng TB (2013) Purification a laccase exhibiting dye decolorizing ability from an edible mushroom Russula virescens. Int Biodeterior Biodegrad 82:33–39
Acknowledgements
This study was financially supported by the NSFC (No. 41171253, No. J1103518) and the National High Technology Research and Development Program of China (No. 2013AA06A210). The authors wish to thank Professor Guanglei Cheng and Dong Yu from Sichuan University for their technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Jaume Bech
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, B., Chen, N. et al. Combined bioremediation of soil co-contaminated with cadmium and endosulfan by Pleurotus eryngii and Coprinus comatus . J Soils Sediments 18, 2136–2147 (2018). https://doi.org/10.1007/s11368-017-1762-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1762-9