Abstract
Purpose
Graphene oxide (GO) nanomaterial has found wide potential industrial applications, but its life cycle environmental impact is not fully understood mainly because of lack of characterization factors (CFs) for the life cycle impact assessment. In this paper, we report the derivation of CF for freshwater ecotoxicity of GO based on the USEtox method.
Methods
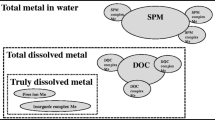
The CF is derived based on the toxic effect factor, fate factor, and exposure factor of GO in the aquatic environment. The toxic effect factor is extracted from mechanistic toxicity studies available in the literature. The fate factor is derived with the colloidal method, and the exposure factor is determined through Langmuir adsorption isotherm for interactions between GO and dissolve organic carbon. Additionally, both fate factor and exposure factor are re-calculated through the default mass-balanced model in USEtox. The apparent octanol-water partition coefficient (K ow) required in the mass balanced model is determined via experiment. Other parameters are calculated according to the apparent K ow.
Results and discussion
The study derives a CF of 777.5 potentially affected species (PAF) day m3 kg−1 for GO with a fate factor of 27.2 days and an exposure factor of 0.93. Sensitivity analysis suggests that variability from the effect factor is the dominant source leading changes in CF. The uncertainty of CF value can vary between ∼1 and 103 PAF day m3 kg−1. Comparison between the colloidal and the mass-balanced models indicates that heteroaggregation may be underestimated by using the apparent partition coefficient, and thus, a much higher estimate of fate factor is obtained from the mass-balanced model. Additionally, empirical formulae in the USEtox to correlate other coefficients with K ow are not proper to calculate bioaccumulation and adsorption with dissolved organic carbon since a virtually a unit exposure factor is obtained.
Conclusion
The derived CFs can be readily incorporated into future toxicity assessment on GO. The fate factor is calculated in the colloidal model while adsorption of dissolved organic carbon onto GO surface should be derived from the Langmuir isotherm. Compared to the colloidal-based method, the conventional mass-balanced method may not be well applicable to GO due to the significant uncertainties in fate and exposure factors from applying the apparent partition coefficients. As three orders of magnitude variations in CF are caused by effect factor due to limited toxicity tests available for GO, more toxicological studies of GO on various species are needed in the future.






Similar content being viewed by others
References
Ahmed F, Rodrigues DF (2013) Investigation of acute effects of graphene oxide on wastewater microbial community: a case study. J Hazard Mater 256:33–39
Arvidsson R, Molander S, Sanden BA, Hassellov M (2011) Challenges in exposure modeling of nanoparticles in aquatic environments. Hum Ecol Risk Assess 17:245–262
Arvidsson R, Kushnir D, Sanden BA, Molander S (2014) Prospective life cycle assessment of graphene production by ultrasonication and chemical reduction. Environ Sci Technol 48:4529–4536
Berenbrock C, Tranmer AW (2008) Simulation of flow, sediment transport, and sediment mobility of the lower Coeur d’Alene River, Idaho. US Geological Survey. http://pubs.usgs.gov/sir/2008/5093/section1.html. Accessed 12 November 2015
Bernard C, Nguyen T, Pellegrin B, Holbrook RD, Zhao M, Chin J (2011) Fate of graphene in polymer nanocomposite exposed to UV radiation. J Phys: Conf Ser 304:012063
Cai L, Zhu J, Hou Y, Tong M, Kim H (2015) Influence of gravity on transport and retention of representative engineered nanoparticles in quartz sand. J Contam Hydrol 181:153–160
Chen D, Feng HB, Li JH (2012a) Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev 112:6027–6053
Chen LQ, Hu PP, Zhang L, Huang SZ, Luo LF, Huang CZ (2012b) Toxicity of graphene oxide and multi-walled carbon nanotubes against human cells and zebrafish. Sci China: Chem 55:2209–2216
Chen Y, Ren C, Ouyang S, Hu X, Zhou Q (2015) Mitigation in multiple effects of graphene oxide toxicity in zebrafish embryogenesis driven by humic acid. Environ Sci Technol 49:10147–10154
Chen Y, Hu X, Sun J, Zhou Q (2016) Specific nanotoxicity of graphene oxide during zebrafish embryogenesis. Nanotoxicology 10:42–52
Chowdhury I, Duch MC, Mansukhani ND, Hersam MC, Bouchard D (2013a) Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ Sci Technol 47:6288–6296
Chowdhury I, Duch MC, Mansukhani ND, Hersam MC, Bouchard D (2013b) Deposition and release of graphene oxide nanomaterials using a quartz crystal microbalance. Environ Sci Technol 48:961–969
Chowdhury I, Hou WC, Goodwin D, Henderson M, Zepp RG, Bouchard D (2015) Sunlight affects aggregation and deposition of graphene oxide in the aquatic environment. Water Res 78:37–46
Compton OC, An Z, Putz KW, Hong BJ, Hauser BG, Brinson LC, Nguyen ST (2012) Additive-free hydrogelation of graphene oxide by ultrasonication. Carbon 50:3399–3406
Cornelis G (2015) Fate descriptors for engineered nanoparticles: the good, the bad, and the ugly. Environ-Sci Nano 2:19–26
Dale AL, Casman EA, Lowry GV, Lead JR, Viparelli E, Baalousha M (2015a) Modeling nanomaterial environmental fate in aquatic systems. Environ Sci Technol 49:2587–2593
Dale AL, Lowry GV, Casman EA (2015b) Much ado about alpha: reframing the debate over appropriate fate descriptors in nanoparticle environmental risk modeling. Environ-Sci Nano 2:27–32
Dong Y, Gandhi N, Hauschild MZ (2014) Development of comparative toxicity potentials of 14 cationic metals in freshwater. Chemosphere 112:26–33
Eckelman MJ, Mauter MS, Isaacs JA, Elimelech M (2012) New perspectives on nanomaterial aquatic ecotoxicity: production impacts exceed direct exposure impacts for carbon nanotubes. Environ Sci Technol 46:2902–2910
Ertürk MD, Saçan MT (2012) First toxicity data of chlorophenols on marine alga Dunaliella tertiolecta: correlation of marine algal toxicity with hydrophobicity and interspecies toxicity relationships. Environ Toxicol Chem 31:1113–1120
Fan W, Jiang X, Lu Y, Huo M, Lin S, Geng Z (2015a) Effects of surfactants on graphene oxide nanoparticles transport in saturated porous media. J Environ Sci 35:12–19
Fan W, Jiang XH, Yang W, Geng Z, Huo MX, Liu ZM, Zhou H (2015b) Transport of graphene oxide in saturated porous media: effect of cation composition in mixed Na–Ca electrolyte systems. Sci Total Environ 511:509–515
Feriancikova L, Xu SP (2012) Deposition and remobilization of graphene oxide within saturated sand packs. J Hazard Mater 235:194–200
Gilbertson LM, Wender BA, Zimmerman JB, Eckelman MJ (2015) Coordinating modeling and experimental research of engineered nanomaterials to improve life cycle assessment studies. Environ-Sci Nano. doi:10.1039/C5EN00097A
Goedkoop M, Heijungs R, Huijbregts M, De Schryver A, Struijs J, Van Zelm R (2009) ReCiPe 2008, a life cycle impact assessment method which comprises harmonised category indicators at the midpoint and the endpoint level; report I: characterisation. http://www.lcia-recipe.net/publications. Accessed 12 November 2015
Golsteijn L, Hendriks HWM, van Zelm R, Ragas AMJ, Huijbregts MAJ (2012) Do interspecies correlation estimations increase the reliability of toxicity estimates for wildlife? Ecotox Environ Safe 80:238–243
Gonzalo R-G, Benedikt Z, Marcel W (2014) Nanotoxicity and life cycle assessment: first attempt towards the determination of characterization factors for carbon nanotubes. IOP Conf Ser: Mater Sci Eng 64:012029
Guo X, Mei N (2014) Assessment of the toxic potential of graphene family nanomaterials. J Food Drug Anal 22(1):105–115
Guo XK, Dong SP, Petersen EJ, Gao SX, Huang QG, Mao L (2013) Biological uptake and depuration of radio-labeled graphene by Daphnia magna. Environ Sci Technol 47:12524–12531
Hartono T, Wang SB, Ma Q, Zhu ZH (2009) Layer structured graphite oxide as a novel adsorbent for humic acid removal from aqueous solution. J Colloid Interf Sci 333:114–119
Hauschild M (2007) GM-troph: a low data demand ecotoxicity effect indicator for use in LCIA. Int J Life Cycle Ass 12:79–91
Hellweg S, Canals LMI (2014) Emerging approaches, challenges and opportunities in life cycle assessment. Science 344:1109–1113
Henderson AD et al (2011) USEtox fate and ecotoxicity factors for comparative assessment of toxic emissions in life cycle analysis: sensitivity to key chemical properties. Int J Life Cycle Assess 16:701–709
Hischier R, Walser T (2012) Life cycle assessment of engineered nanomaterials: state of the art and strategies to overcome existing gaps. Sci Total Environ 425:271–282
Hou WC, Westerhoff P, Posner JD (2013) Biological accumulation of engineered nanomaterials: a review of current knowledge. Environ Sci Process Impacts 15:103–122
Hou W-C, Chowdhury I, Goodwin DG, Henderson WM, Fairbrother DH, Bouchard D, Zepp RG (2015) Photochemical transformation of graphene oxide in sunlight. Environ Sci Technol 49:3435–3443
Hu X, Mu L, Kang J, Lu K, Zhou R, Zhou Q (2014) Humic acid acts as a natural antidote of graphene by regulating nanomaterial translocation and metabolic fluxes in vivo. Environ Sci Technol 48:6919–6927
Hu X, Ouyang S, Mu L, An J, Zhou Q (2015) Effects of graphene oxide and oxidized carbon nanotubes on the cellular division, microstructure, uptake, oxidative stress, and metabolic profiles. Environ Sci Technol 49:10825–10833
Hua Z, Tang Z, Bai X, Zhang J, Yu L, Cheng H (2015) Aggregation and resuspension of graphene oxide in simulated natural surface aquatic environments. Environ Pollut 205:161–169
Kanakia S et al (2013) Physicochemical characterization of a novel graphene-based magnetic resonance imaging contrast agent. Int J Nanomedicine 8:2821–2833
Konios D, Stylianakis MM, Stratakis E, Kymakis E (2014) Dispersion behaviour of graphene oxide and reduced graphene oxide. J Colloid Interf Sci 430:108–112
Lanphere JD, Luth CJ, Walker SL (2013) Effects of solution chemistry on the transport of graphene oxide in saturated porous media. Environ Sci Technol 47:4255–4261
Li J, Zhang S, Chen C, Zhao G, Yang X, Li J, Wang X (2012) Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. Acs Appl Mater Inter 4:4991–5000
Li S, Pan X, Wallis LK, Fan Z, Chen Z, Diamond SA (2014) Comparison of TiO2 nanoparticle and graphene–TiO2 nanoparticle composite phototoxicity to Daphnia magna and Oryzias latipes. Chemosphere 112:62–69
Liu HH, Cohen Y (2014) Multimedia environmental distribution of engineered nanomaterials. Environ Sci Technol 48:3281–3292
Liu XT et al (2014) Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos. Biomed Environ Sci 27:676–683
Maes HM, Stibany F, Giefers S, Daniels B, Deutschmann B, Baumgartner W, Schaffer A (2014) Accumulation and distribution of multiwalled carbon nanotubes in zebrafish (Danio rerio). Environ Sci Technol 48:12256–12264
Meesters JAJ, Koelmans AA, Quik JTK, Hendriks AJ, van de Meentt D (2014) Multimedia modeling of engineered nanoparticles with SimpleBox4nano: model definition and evaluation. Environ Sci Technol 48:5726–5736
Mesaric T, Sepcic K, Piazza V, Gambardella C, Garaventa F, Drobne D, Faimali M (2013) Effects of nano carbon black and single-layer graphene oxide on settlement, survival and swimming behaviour of Amphibalanus amphitrite larvae. Chem Ecol 29:643–652
Miseljic M, Olsen SI (2014) Life-cycle assessment of engineered nanomaterials: a literature review of assessment status. J Nanopart Res 16:2427. doi:10.1007/S11051-014-2427-X
Nogueira PFM, Nakabayashi D, Zucolotto V (2015) The effects of graphene oxide on green algae Raphidocelis subcapitata. Aquat Toxicol 166:29–35
Pan B, Xing BS (2008) Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ Sci Technol 42:9005–9013
Payet J (2004) Assessing toxic impacts on aquatic ecosystems in life cycle assessment (LCA). Dissertation, École polytechnique fédérale de Lausanne
Petersen EJ, Akkanen J, Kukkonen JVK, Weber WJ (2009) Biological uptake and depuration of carbon nano-tubes by Daphnia magna. Environ Sci Technol 43:2969–2975
Praetorius A, Scheringer M, Hungerbuhler K (2012) Development of environmental fate models for engineered nanoparticles—a case study of TiO2 nanoparticles in the Rhine River. Environ Sci Technol 46:6705–6713
Praetorius A, Tufenkji N, Goss K-U, Scheringer M, von der Kammer F, Elimelech M (2014) The road to nowhere: equilibrium partition coefficients for nanoparticles. Environ-Sci Nano 1:317–323
Pretti C et al (2014) Ecotoxicity of pristine graphene to marine organisms. Ecotox Environ Safe 101:138–145
Qi ZC, Zhang LL, Wang F, Hou L, Chen W (2014) Factors controlling transport of graphene oxide nanoparticles in saturated sand columns. Environ Toxicol Chem 33:998–1004
Quik JTK, Vonk JA, Hansen SF, Baun A, Van De Meent D (2011) How to assess exposure of aquatic organisms to manufactured nanoparticles? Environ Int 37:1068–1077
Quik JTK, van de Meent D, Koelmans AA (2014a) Simplifying modeling of nanoparticle aggregation-sedimentation behavior in environmental systems: a theoretical analysis. Water Res 62:193–201
Quik JTK, Velzeboer I, Wouterse M, Koelmans AA, van de Meent D (2014b) Heteroaggregation and sedimentation rates for nanomaterials in natural waters. Water Res 48:269–279
Quik JTK, de Klein JJM, Koelmans AA (2015) Spatially explicit fate modelling of nanomaterials in natural waters. Water Res 80:200–208
Radix P et al (2000) Comparison of four chronic toxicity tests using algae, bacteria, and invertebrates assessed with sixteen chemicals. Ecotox Environ Safe 47:186–194
Roelofs W, Huijbregts MAJ, Jager T, Ragas AMJ (2003) Prediction of ecological no-effect concentrations for initial risk assessment: combining substance-specific data and database information. Environ Toxicol Chem 22:1387–1393
Roex EWM, de Vries E, van Gestel CAM (2002) Sensitivity of the zebrafish (Danio rerio) early life stage test for compounds with different modes of action. Environ Pollut 120:355–362
Rosenbaum RK, Margni M, Jolliet O (2007) A flexible matrix algebra framework for the multimedia multipathway modeling of emission to impacts. Environ Int 33:624–634
Rosenbaum R et al (2008) USEtox-the UNEP-SETAC toxicity model: recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int J Life Cycle Ass 13:532–546
Rosenkranz PW (2010) The ecotoxicology of nanoparticles in Daphnia magna. Dissertation, University of Edinburgh
Rossetto AL, Melegari SP, Ouriques LC, Matias WG (2014) Comparative evaluation of acute and chronic toxicities of CuO nanoparticles and bulk using Daphnia magna and Vibrio fischeri. Sci Total Environ 490:807–814
Salieri B, Righi S, Pasteris A, Olsen SI (2015) Freshwater ecotoxicity characterisation factor for metal oxide nanoparticles: a case study on titanium dioxide nanoparticle. Sci Total Environ 505:494–502
Sanchez VC, Jackhak A, Hurt RH, Kane AB (2012) Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem Res Toxicol 25:15–34
Seabra AB, Paula AJ, de Lima R, Alves OL, Durán N (2014) Nanotoxicity of graphene and graphene oxide. Chem Res Toxicol 27:159–168
Sotirelis NP, Chrysikopoulos CV (2015) Interaction between graphene oxide nanoparticles and quartz sand. Environ Sci Technol. doi:10.1021/acs.est.5b03496
Soulsby R (1997) Dynamics of marine sands: a manual for practical applications. Thomas Telford, London
Susfalk RB, Fitzgerald B, Knust AM (2008) Characterization of turbidity and total suspended solids in the Upper Carson River, Nevada DHS Publication. https://ndep.nv.gov/bwqp/file/41242-07_upper_carson.pdf. Accessed 12 November 2015
Thurman EM (1985) Aquatic humic substances. In: Organic geochemistry of natural waters, vol 2. Developments in biogeochemistry. Springer Netherlands, pp 273–361. doi:10.1007/978-94-009-5095-5_11
Tufenkji N, Elimelech M (2004) Correlation equation for predicting single-collector efficiency in physicochemical filtration in saturated porous media. Environ Sci Technol 38:529–536
Walter J, Nacken TJ, Damm C, Thajudeen T, Eigler S, Peukert W (2015) Determination of the lateral dimension of graphene oxide nanosheets using analytical ultracentrifugation. Small 11:814–825
Wang A, Pu K, Dong B, Liu Y, Zhang L, Zhang Z, Duan W, Zhu Y (2013) Role of surface charge and oxidative stress in cytotoxicity and genotoxicity of graphene oxide towards human lung fibroblast cells. J Appl Toxicol 33:1156–1164
Wang Z, Quik JTK, Song L, Van den Brandhof EJ, Wouterse M, Peijnenburg WJGM (2015) Humic substances alleviate the aquatic toxicity of polyvinylpyrrolidone-coated silver nanoparticles to organisms of different trophic levels. Environ Toxicol Chem 34:1239–1245
Wheeler JR, Leung KMY, Morritt D, Sorokin N, Rogers H, Toy R, Holt M, Whitehouse P, Crane M (2002) Freshwater to saltwater toxicity extrapolation using species sensitivity distributions. Environ Toxicol Chem 21:2459–2467
Wu L et al (2013) Aggregation kinetics of graphene oxides in aqueous solutions: experiments, mechanisms, and modeling. Langmuir 29:15174–15181
Yang S et al (2014) Effects of humic acid on copper adsorption onto few-layer reduced graphene oxide and few-layer graphene oxide. Carbon 75:227–235
Zhang W, Crittenden J, Li K, Chen Y (2012a) Attachment efficiency of nanoparticle aggregation in aqueous dispersions: modeling and experimental validation. Environ Sci Technol 46:7054–7062
Zhang X, Hu W, Li J, Tao L, Wei Y (2012b) A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicol Res 1:62–68
Zhao J, Wang ZY, White JC, Xing BS (2014) Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation. Environ Sci Technol 48:9995–10009
Zhao J, Liu F, Wang Z, Cao X, Xing B (2015) Heteroaggregation of graphene oxide with minerals in aqueous phase. Environ Sci Technol 49:2849–2857
Acknowledgments
The authors thank Dr. Huabing Sun and Professor Xiaohua Peng from the Department of Chemistry, the University of Wisconsin-Milwaukee, for their experimental assistance in determining the octanol-partition coefficient.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ralph K. Rosenbaum
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 680 kb)
Rights and permissions
About this article
Cite this article
Deng, Y., Li, J., Qiu, M. et al. Deriving characterization factors on freshwater ecotoxicity of graphene oxide nanomaterial for life cycle impact assessment. Int J Life Cycle Assess 22, 222–236 (2017). https://doi.org/10.1007/s11367-016-1151-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-016-1151-4