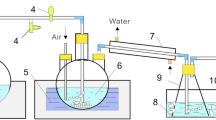
Abstract
A series of experiments of column leaching under different pHs (pH 1.8, 3.8, 6.5, and 8.5) and calcination at different temperatures (200–1100 °C) were carried out for evaluation of release behavior of iodine in phosphate rock. The modes of occurrence of iodine in the phosphate rock and its leaching and calcination residues were extracted with sequential chemical extraction. Iodine in solution and solid samples was measured with ion chromatography (IC) and pyrohydrolysis combined ion chromatography (PIC), respectively. Mineralogical compositions of phosphate rock and the leached and calcined residues were determined by XRD (X-ray diffraction) and FT-IR (Fourier infrared spectrum). The results show that iodine in phosphate rock occurred in a descending order of significance, as forms of residual, carbonate bound, ion-exchange, organic bound, Fe-Mn oxide bound, and water soluble. Under pH 1.8, 3.8, 6.5, and 8.5, the release iodine may almost reach the maximum at the leaching time of 65, 93, 90, and 165 h, with leaching rates of 5.28%, 1.24%, 0.550%, and 1.08% and the average iodine concentrations in the leachates of 2300 μg/L, 378 μg/L, 164 μg/L, and 189 μg/L, respectively. The forms of the leached iodine were mostly ion-exchange and carbonate-bound iodine under pH 1.8 and water soluble and ion-exchange iodine under pH 3.8, 6.5, and 8.5. By calcination, the total iodine was released rapidly in 200–300 °C and 700–1000 °C, and almost released completely at 1000 °C, with a leaching rate of 96.6%. The ion-exchange and organic-bound iodine were, respectively, released at 200–1000 °C and at less than 300 °C; the carbonate-bound and residual iodine were mainly released at more than 700 °C. The release iodine in phosphate rock leached by natural water and calcined at a high temperature may lead to the increase of iodine concentration of water body and atmosphere.










Similar content being viewed by others
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alicke B, Hebestreit K, Stutz J, Platt U (1999) Iodine oxide in the marine boundary layer. Nature 397:572–573
Claret F, Lerouge C, Laurioux T, Bizi M, Conte T, Ghestem JP, Wille G, Sato T, Gaucher EC, Giffaut E, Tournassat C (2010) Natural iodine in a clay formation: Implications for iodine fate in geological disposals. Geochim Cosmochim Ac 74:16–29
Gao X, Hu Y, Li C, Dai C, Li L, Ou X, Wang Y (2016) Evaluation of fluorine release from air deposited coal spoil piles: a case study at Yangquan city, northern China. Sci Total Environ 545-546:1–10
Golda M, Janas A, Olszewska D (2011) The leaching of chlorine from hard coal Part I. Relationship between the process parameters and its effectivity. Fuel Process Technol 92:1230–1235
Gong D, Zhou K, Peng C, Li J, Chen W (2019) Sequential extraction of tungsten from scheelite through roasting and alkaline leaching. Miner Eng 132:238–244
Guo F, Li J (2011) Selective separation of silica from a siliceouse-calcareous phosphate rock. Min Sci Technol 21:135–139
Hosseini SA, Raygan S, Rezaei A, Jafari A (2017) Leaching of nickel from a secondary source by sulfuric acid. J Environ Chem Eng 5:3922–3929
Kaplan DI, Serne RJ, Parker KE, Igor V, Kutnyakov IV (2000) Iodide sorption to subsurface sediments and illitic minerals. Environ Sci Technol 34:399–405
Komnitsas K, Petrakis E, Bartzas G, Karmali V (2019) Column leaching of low-grade saprolitic laterites and valorization of leaching residues. Sci Total Environ 665:347–357
Lei X (2013) Primary study on occurrence state and recovery methods of iodine in phosphate rock. Phosph Co Fertil 28:58–60 (in Chinese)
Liu L, Wu D, Li P (2010) Iodine emission during the soil baking process and its environmental significance. Earth Environ 38:439–443 (in Chinese)
Liu Y, Zhang Y, Yu W, Zhang S, Liu X, Zhang S (2014) The analysis of resource and environmental constraints of China’s phosphate resources demand situation. China Min Mag 23:1–5 (in Chinese)
Luo M, Hou X, Zhou W, He C, Chen N, Liu Q, Zhang L (2013) Speciation and migration of 129I in soil profiles. J Environ Radioact 118:30–39
Maes S, Zhuang W, Rabaey K, Alvarez-Cohen L, Hennebel T (2017) Concomitant leaching and electrochemical extraction of rare earth elements from monazite. Environ Sci Technol 51:1654–1661
Mao X, Lu Q, Mo W, Xin X, Chen X, He Z (2017) Phosphorus availability and release pattern from activated dolomite phosphate rock in Central Florida. J Agric Food Chem 65:4589–4596
Nie Y, Li S, Wu C, Wang C, He D, Mei Y (2018) Efficient removal of SO2 from flue gas with phosphate rock slurry and investigation of reaction mechanism. Ind Eng Chem Res 57:15138–15146
Ntakamutshi PT, Kime M, Mwema ME, Ngenda BR, Kaniki TA (2017) Agitation and column leaching studies of oxidised copper-cobalt ores under reducing conditions. Miner Eng 111:47–54
Peng B, Li X, Zhao W, Yang L (2018) Study on the release characteristics of chlorine in coal gangue under leaching conditions of different pH values. Fuel 217:427–433
Peng B, Wu D (2011) Leaching characteristics of bromine in coal. J Fuel Chem Technol 39:647–651
Peng B, Wu D (2012) Modes of iodine occurrence in bituminous coal and anthracite and their environmental effects. J Fuel Chem Technol 40:257–262
Peng B, Wu D (2013a) Simultaneous rapid determination of halogens in clay using pyrohydrolysis combined with Ion chromatography. Chin J Anal Chem 41:1499–1504
Peng B, Wu D (2013b) Leaching characteristics of iodine in coal. J Fuel Chem Technol 41:129–133 (in Chinese)
Ren H, Yang R, Gao J, Cheng W, We H (2015) Enrichment regularities for iodine concentration in phosphorite of Doushantuo Formation of Late Ediacaran in Guizhou Province. SW China Arab J Geosci 8:5423–5437
Roulier M, Bueno M, Thiry Y, Coppin F, Redon PO, Hécho IL, Pannier F (2018) Iodine distribution and cycling in a beech (Fagus sylvatica) temperate forest. Sci Total Environ 645:431–440
Sarwar G, Gantt B, Foley K, Fahey K, Saiz-Lopez A (2019) Influence of bromine and iodine chemistry on annual, seasonal, diurnal, and background ozone: CMAQ simulations over the Northern Hemisphere. Atmos Environ 213:395–404
Sınırkaya M, Özer AK, Gülaboglu MS (2011) Investigation of the changes of P2O5 content of phosphate rock during simultaneous calcination/sulfation. Powder Technol 211:72–76
Sun K, She S, Wang K, Chen X (2009) Study on phase structure and occurrence of iodine in phosphate ores from Wengfu Mine in Guizhou. Ind Miner Process 10:17–19 (in Chinese)
Szilas C, Bender KC, Msolla MM, Borggaard OK (2008) The reactivity of Tanzanian Minjingu phosphate rock can be assessed from the chemical and mineralogical composition. Geoderma 147:172–177
Torrisi C (2001) Leaching of fluorine bearing minerals from lead and zinc concentrates. Miner Eng 14:1637–1648
Vaccari DA, Powers SM, Liu X (2019) Demand-driven model for global phosphate rock suggests paths for phosphorus sustainability. Environ Sci Technol 53:10417–10425
Wang S, Luo K, Wang X, Sun Y (2016) Estimate of sulfur, arsenic, mercury, fluorine emissions due to spontaneous combustion of coal gangue: an important part of Chinese emission inventories. Environ Pollut 209:107–113
Wu D, Deng H, Zheng B, Wang W, Tang X, Xiao H (2008) Iodine in Chinese coals and its geochemistry during coalification. Appl Geochem 23:2082–2090
Wu D, Du J, Deng H, Wang W, Xiao H, Li P (2014) Estimation of atmospheric iodine emission from coal combustion. Int J Environ Sci Technol 11:357–366
Xing B, Chen T, Liu H, Qing C, Xie J, Xie Q (2017) Removal of phosphate from aqueous solution by activated siderite ore: preparation, performance and mechanism. J Taiwan Inst Chem Eng 80:875–882
Zhang S, Xu C, Creeley D, Ho YF, Li HP, Grandbois R, Schwehr KA, Kaplan DI, Yeager CM, Wellman D, Santschi PH (2013) Iodine-129 and Iodine-127 speciation in groundwater at the Hanford Site, U.S.: iodate incorporation into calcite. Environ Sci Technol 47:9635–9642
Zhang Z, Gustin L, Xie W, Lian J, Kalliat T, Valsaraj KT, Wang J (2019) Effect of solution chemistry on the iodine release from iodoapatite in aqueous environments. J Nucl Mater 525:161–170
Zhang Z, Heath A, Valsaraj KT, Ebert WL, Yao T, Lian J, Wang J (2018) Mechanism of iodine release from iodoapatite in aqueous solution. RSC Adv 8:3951–3957
Zhou F, Yang L, Zhang L, Cao J, Zhang H (2019) Investigation of decomposition of dolomite and distribution of iodine migration during the calcination-digestion process of phosphate ore. Hydrometallurgy 188:174–181
Funding
This work was sponsored by National Natural Science Foundation of China (Grant No. 21966014), Graduate Innovation Fund of Jiangxi Normal University (YJS2019018), and Science and Technology Research Project from Educational Department of Jiangxi Province in China (GJJ170173).
Author information
Authors and Affiliations
Contributions
Bingxian Peng: Methodology, sampling, experiment, data processing, and writing.
Xinrui Li: Experiment and data processing.
Sulin Xiang: Sampling and investigation.
Linyan Lei: Sampling and experiment.
Mengqi Yang: Sampling and experiment.
Lei Zhu: Experiment.
Yang Qi: Data processing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, B., Li, X., Xiang, S. et al. Release behavior of iodine during leaching and calcination of phosphate rock. Environ Sci Pollut Res 28, 31059–31070 (2021). https://doi.org/10.1007/s11356-021-12895-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12895-w