Abstract
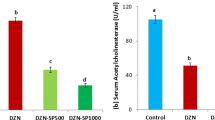
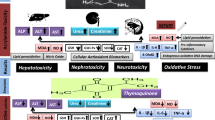
Acrylamide (AA) is a hazardous chemical that is widely used in industrial practices. Spirulina platensis (SP) is a blue green alga that is rich in bioactive compounds with many medicinal benefits. The aim of the present study was to evaluate the ameliorative effect of SP against AA toxicity in rats. Animals were divided into six groups: Group (1) was normal rats, groups (2) and (3) received SP at 500 and 1000 mg/kg BW orally respectively for 21 days, group (4) was administered 20 mg/kg BW AA daily for 14 days, while groups (5) and (6) were given orally SP at the same doses of groups (2) and (3), then AA at similar dose of group (4). Rats that received AA alone displayed markedly increased serum levels of liver enzymes (ALT, AST, and ALP), kidney function parameters (urea and creatinine), DNA damage marker (8-OHdG), and proinflammatory cytokines (IL-1β, IL-6, and TNF-α), compared to control rats. Furthermore, tissue analysis revealed marked increases in hepatic, renal, and brain MDA and NO, as well as marked reductions in the antioxidant biomarkers (GSH, GSH-Px, SOD, and CAT) in acrylamide-intoxicated rats. Spirulina ameliorated the alterations in serum biochemical parameters and reduced MDA and NO, as well as improved antioxidant biomarkers in AA-intoxicated rats in a dose-dependent manner. Our results show that SP has a powerful protective effect on serum biochemistry and liver, kidney, and brain antioxidant machinery in AA-intoxicated rats.





Similar content being viewed by others
Data availability
All data are available from the corresponding author when required.
Abbreviations
- AA:
-
acrylamide
- ALT:
-
alanine aminotransferase
- ALP:
-
alkaline phosphatase
- ANOVA:
-
analysis of variance
- AST:
-
aspartate aminotransferase
- BW:
-
body weight
- °C:
-
degree Celsius
- CAT:
-
catalase
- CCl4:
-
carbon tetrachloride
- DNA:
-
deoxyribonucleic acid
- ELISA:
-
enzyme-linked immunosorbent assay
- g:
-
gram
- GSH:
-
reduced glutathione
- GSH-Px:
-
glutathione peroxidase
- h:
-
hour
- IL:
-
interleukin
- kg:
-
kilogram
- MDA:
-
malondialdehyde
- mg:
-
milligram
- min:
-
minute
- mM:
-
millimolar
- NO:
-
nitric oxide
- Na2HPO4 :
-
disodium hydrogen phosphate
- NaH2PO4 :
-
sodium dihydrogen phosphate
- P:
-
probability
- pH:
-
potential of hydrogen
- ROS:
-
reactive oxygen species
- rpm:
-
revolutions per minute
- SEM:
-
standard error of the mean
- SOD:
-
superoxide dismutase
- SP :
-
Spirulina platensis
- SPSS:
-
Statistical Package for Social Sciences
- TNF-α:
-
tumor necrosis factor-alpha
- 8-OHdG:
-
8-hydroxy-2′-deoxyguanosine
- β:
-
beta
References
Abdel-Daim MM (2014) Pharmacodynamic interaction of Spirulina platensis with erythromycin in Egyptian Baladi bucks (Capra hircus). Small Ruminant Research 120:234–241
Abdel-Daim MM, Abuzead SM, Halawa SM (2013) Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. Plos one 8:e72991
Abdel-Daim MM, Farouk SM, Madkour FF, AZAB SS (2015) Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol Immunotoxicol 37:126–139
Abdel-Daim M, El-Bialy BE, Rahman HG, Radi AM, Hefny HA, Hassan AM (2016 Feb) Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: biochemical and histopathological studies. Biomed Pharmacother. 77:79–85. https://doi.org/10.1016/j.biopha.2015.12.003
Abdel-Daim MM, Abushouk AI, Alkhalf MI, Toraih EA, Fawzy MS, Ijaz H, Aleya L, Bungau SG (2018 Sep) Antagonistic effects of Spirulina platensis on diazinon-induced hemato-biochemical alterations and oxidative stress in rats. Environ Sci Pollut Res Int. 25(27):27463–27470. https://doi.org/10.1007/s11356-018-2761-0
Abdel-Daim MM, Dawood MAO, Elbadawy M, Aleya L, Alkahtani S (2020a) Spirulina platensis reduced oxidative damage induced by chlorpyrifos toxicity in Nile tilapia (Oreochromis niloticus). Animals 10:473
Abdel-Daim MM, Dawood MAO, AlKahtane AA, Abdeen A, Abdel-Latif HMR, Senousy HH, Aleya L, Alkahtani S (2020b Sep) Spirulina platensis mediated the biochemical indices and antioxidative function of Nile tilapia (Oreochromis niloticus) intoxicated with aflatoxin B1. Toxicon. 184:152–157. https://doi.org/10.1016/j.toxicon.2020.06.001
Abdelkhalek NK, Ghazy EW, Abdel-Daim MM (2015 Feb) Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: impact on lipid peroxidation and oxidative stress. Environ Sci Pollut Res Int. 22(4):3023–3031. https://doi.org/10.1007/s11356-014-3578-0
Abdelkhalek NKM, Eissa IAM, Ahmed E, Kilany OE, El-Adl M, Dawood MAO, Hassan AM, Abdel-Daim MM (2017 Sep) Protective role of dietary Spirulina platensis against diazinon-induced oxidative damage in Nile tilapia. Oreochromis niloticus. Environ Toxicol Pharmacol. 54:99–104. https://doi.org/10.1016/j.etap.2017.07.002
AEBI H (1984) Catalase in Vitro. Methods Enzymol 105:121–126
Avdagic N, Cosovic E, Nakas-Icindic E, Mornjakovic Z, Zaciragic A, Hadzovic-Dzuvo A (2008) Spirulina platensis protects against renal injury in rats with gentamicin-induced acute tubular necrosis. Bosn J Basic Med Sci 8:331–336
Babizhayev MA (2016) Generation of reactive oxygen species in the anterior eye segment. Synergistic codrugs of N-acetylcarnosine lubricant eye drops and mitochondria-targeted antioxidant act as a powerful therapeutic platform for the treatment of cataracts and primary open-angle glaucoma. BBA Clin 6:49–68
Benitez, C., Lucas, A., Aispur, G., Álvarez, M., Llanos, C., Quintana, M. A. & Brandan, N. 2004. Efectos De Un Potencial Hepatotóxico: La Acrilamida Monomérica, En Un Modelo Murino. Comunicaciones científicas y tecnológicas.
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Capuano E, Fogliano V (2011) Acrylamide and 5-hydroxymethylfurfural (Hmf): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT-food science and technology 44:793–810
Chamorro, G., Salazar, M., Araujo, K. G., dos Santos, C. P., Ceballos, G. & Castillo, L. F. 2002. Update on the pharmacology of Spirulina (Arthrospira), an unconventional food. Arch Latinoam Nutr, 52, 232-240.
Coulombe JJ, Favreau L (1963) A new simple semimicro method for colorimetric determination of urea. Clin Chem 9:102–108
El-Beltagi HS, Ahmed MM (2016) Assessment the protective role of quercetin on acrylamide-induced oxidative stress in rats. Journal of Food Biochemistry 40:715–723
Farooq SM, Asokan D, Kalaiselvi P, Sakthivel R, Varalakshmi P (2004) Prophylactic role of phycocyanin: a study of oxalate mediated renal cell injury. Chem Biol Interact 149:1–7
GargourI, M., Soussi, A., Akrouti, A., Magne, C. & El Feki, A. 2018. Ameliorative effects of Spirulina platensis against lead-induced nephrotoxicity in newborn rats: modulation of oxidative stress and histopathological changes. Excli j, 17, 215-232.
Green l C, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15n] nitrate in biological fluids. Analytical biochemistry 126:131–138
Guyton AC, Hall JE (2006) Textbook of medical physiology 11th Ed. Elsiever Saunders. In: 788-817
Kahkeshani N, Saeidnia S, Abdollahi M (2015) Role of antioxidants and phytochemicals on acrylamide mitigation from food and reducing its toxicity. J Food Sci Technol 52:3169–3186
Kepekci RA, Polat S, Celik A, Bayat N, Saygideger SD (2013) Protective effect of Spirulina platensis enriched in phenolic compounds against hepatotoxicity induced by Ccl4. Food Chem 141:1972–1979
Klaunig JE (2008) Acrylamide carcinogenicity. J Agric Food Chem 56:5984–5988
Kumar N, Singh S, Patro N, Patro I (2009) Evaluation of protective efficacy of Spirulina platensis against collagen-induced arthritis in rats. Inflammopharmacology 17:181–190
Larsen K (1972) Creatinine assay by a reaction-kinetic principle. Clin Chim Acta 41:209–217
Liu Z, Song G, Zou C, Liu G, Wu W, Yuan T, Liu X (2015) Acrylamide induces mitochondrial dysfunction and apoptosis in Bv-2 microglial cells. Free Radical Biology and Medicine 84:42–53
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Nita M, Grzybowski A (2016) The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Medicine and Cellular Longevity 2016:1–23
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pak W, Takayama F, Mine M, Nakamoto K, Kodo Y, Mankura M, Egashira T, Kawasaki H, Mori A (2012) Anti-oxidative and anti-inflammatory effects of Spirulina on rat model of non-alcoholic steatohepatitis. J Clin Biochem Nutr 51:227–234
Pennisi M, Malaguarnera G, Puglisi V, Vinciguerra L, Vacante M, Malaguarnera M (2013) Neurotoxicity of acrylamide in exposed workers. Int J Environ Res Public Health 10:3843–3854
Pradeep K, Mohan CV, Gobianand K, Karthikeyan S (2007) Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol 560:110–116
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Rivadeneyra-Dominguez E, Becerra-Contreras Y, Vazquez-Luna A, Diaz-Sobac R, Rodriguez-Landa JF (2018) Alterations of blood chemistry, hepatic and renal function, and blood cytometry in acrylamide-treated rats. Toxicol Rep 5:1124–1128
Salzano S, Checconi P, Hanschmann E-M, Lillig CH, Bowler LD, Chan P, Vaudry D, Mengozzi M, Coppo L, Sacre S (2014) Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proceedings of the National Academy of Sciences 111:12157–12162
Szczerbina T, Banach Z, Tylko G, Pyza E (2008) Toxic effects of acrylamide on survival, development and haemocytes of Musca domestica. Food Chem Toxicol 46:2316–2319
Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD, Shuey D, Zygowicz ER (1983) A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem 29:751–761
Wu Q, Liu L, Miron A, Klimova B, Wan D, Kuca K (2016) The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol 90:1817–1840
Yousef MI, El-Demerdash FM (2006) Acrylamide-induced oxidative stress and biochemical perturbations in rats. Toxicology 219:133–141
Yuliana ND, Khatib A, Link-Struensee AM, Ijzerman AP, Rungkat-Zakaria F, Choi YH, Verpoorte R (2009) Adenosine A1 receptor binding activity of methoxy flavonoids from Orthosiphon stamineus. Planta Med 75:132–136
Zhang L, Wang E, Chen F, Yan H, Yuan Y (2013) Potential protective effects of oral administration of allicin on acrylamide-induced toxicity in male mice. Food & function 4:1229–1236
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number PNU-DRI-RI-20-009.
Funding
The Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia funded this research through project number PNU-DRI-RI-20-009.
Author information
Authors and Affiliations
Contributions
Idea and design: M.B-J., A.A.A., N. A., M. K., M.M.A.F., M.M.A.A., I.M.S., M. M. A-D.
Data collection: M.B-J., A.A.A., N. A., M. K., M.M.A.F., M.M.A.A., I.M.S., M. M. A-D.
Data analysis: M.B-J., A.A.A., N. A., M. K., M.M.A.F., M.M.A.A., I.M.S., M. M. A-D.
Funding: M.B-J., M. M. A-D.
Investigation: M.B-J., A.A.A., N. A., M. K., M.M.A.F.
Methodology: M.B-J., A.A.A., N. A., M. K., M.M.A.F.
Project administration: M.B-J., M. M. A-D.
Software: N. A., M. K., M.M.A.F., M.M.A.A., I.M.S., M. M. A-D.
Supervision: M.B-J., M. M. A-D.
Validation: N. A., M. K., M.M.A.F., M.M.A.A., I.M.S., M. M. A-D.
Visualization: N. A., M. K., M.M.A.F., M.M.A.A., I.M.S., M. M. A-D.
Manuscript draft writing: M.B-J., A.A.A., N. A., M. K., M.M.A.F., M.M.A.A., I.M.S., M. M. A-D.
Manuscript revision and editing: M.M.A.A., I.M.S., M. M. A-D.
All the authors approved and confirmed this submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethics approval
The Research Ethical Committee of the Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt, permitted the experimental scheme and animal management (approval no. 2020042). We took all the possible procedures to reduce rat sufferance.
Consent to participate
Not applicable as the present study did not include human subject.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bin-Jumah, M.N., AL-Huqail, A.A., Abdelnaeim, N. et al. Potential protective effects of Spirulina platensis on liver, kidney, and brain acrylamide toxicity in rats. Environ Sci Pollut Res 28, 26653–26663 (2021). https://doi.org/10.1007/s11356-021-12422-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12422-x