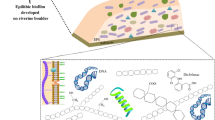
Abstract
Active functional groups in biofilms determine the adsorption and desorption of contaminants and nutrients. Epilithic biofilms were characterized in order to understand the association between the chemistry alteration patterns and the surrounding anthropic activities of the Guaporé River watershed. The instrumental analyses included pyrolysis coupled to gas chromatography and mass spectroscopy, spectroscopy in the IR region with attenuated total reflectance, and two-dimensional nuclear magnetic resonance. Spectrometric techniques demonstrated that epilithic biofilms are mainly composed of polysaccharides, nitrogen-containing compounds, lipids, and aromatic and phenolic structures, which have functional groups characteristic of alcohols, esters, ethers, and amides. The polysaccharide levels reflect well the environmental pressures. The chemical composition of epilithic biofilms can be an effective tool for environmental assessment in watercourses, since the different anthropic actions developed in watersheds, mainly agriculture and urban areas, can modify the organic fraction of biofilms.









Similar content being viewed by others
Data availability
All data generated during this study are included in this published article and its supplementary information files. Additional data are available from the corresponding author on reasonable request.
References
Archela E, Carraro A, Fernandes F, Barros ONF, Archela RS (2003) Considerações sobre a geração de efluentes líquidos em centros urbanos. Geografia 12:517–525 (in Portuguese)
Aubertheau E, Stalder T, Mondamert L, Ploy MC, Dagot C, Labanowski J (2017) Impact of wastewater treatment plant discharge on the contamination of river biofilms by pharmaceuticals and antibiotic resistance. Sci Total Environ 579:1387–1398
Barbosa LCA (2007) Espectroscopia no infravermelho: na caracterização de compostos orgânicos. Ed. UFV, Viçosa, Brasil (in Portuguese)
Bastos MC (2017) Study of the environmental contamination of human and veterinary medicines in the south Brazil. Thesis, Universidade Federal de Santa Maria and École Doctorale Gay Lussac da Université de Poitiers
Bastos MC, Rheinheimer dos Santos D, Monteiro de Castro Lima JA, Le Guet T, Santanna dos Santos MA, Zanella R, Aubertheau E, Mondamert L, Caner L, Labanowski J (2018) Presence of anthropogenic markers in water: a case study of the Guaporé River watershed, Brazil. Clean - Soil Air Water 46:1700019
Bhuyan MS, Bakar MA, Akhtar A, Hossain MB, Ali MM, Islam MS (2017) Heavy metal contamination in surface water and sediment of the Meghna River, Bangladesh. Environ Nanotechnol Monit Manag 8:273–279
Blanco Y, Rivas LA, González-Toril E, Ruiz-Bermejo M, Moreno-Paz M, Parro V, Palacín A, Aguilera A, Puente-Sánchez F (2019) Environmental parameters, and not phylogeny, determine the composition of extracellular polymeric substances in microbial mats from extreme environments. Sci Total Environ 650:384–393
Bonnineau C, Artigas J, Chaumet B, Dabrin A, Faburé J, Ferrari BJD, Lebrun JD, Margoum C, Mazzella N, Miège C, Morin S, Uher E, Babut M, Pesce S (2020) Role of biofilms in contaminant bioaccumulation and trophic transfer in aquatic ecosystems: current state of knowledge and futurechallenges. In: Reviews of Environmental Contamination and Toxicology (Continuation of Residue Reviews). Springer, New York, pp 1–39. https://doi.org/10.1007/398_2019_39
Branda SS, Vik Å, Friedman L, Kolter R (2005) Biofilms: the matrix revisited. Trends Microbiol 13:20–26
Bruchet A, Rousseau C, Mallevialle J (1990) Pyrolysis-GC-MS for investigating high-molecular-weight THM precursors and other refractory organics. J - Am Water Works Assoc 82:66–74
Castro Lima JAM, Labanowski J, Bastos MC et al (2020) “Modern agriculture” transfers many pesticides to watercourses: a case study of a representative rural catchment of southern Brazil. Environ Sci Pollut Res 27:10581–10598
Catter KM, Cavalcante RM, Barreto NSE, Saker-Sampaio S, Hofer E (2007) Bactérias isoladas de mangues do rio Cocó e do riacho das Guaribas (CE) e seu potencial na degradação de derivados e constituintes de petróleo. Geochim Bras 21:140–150 (in Portuguese)
Chonova T, Labanowski J, Cournoyer B, Chardon C, Keck F, Laurent É, Mondamert L, Vasselon V, Wiest L, Bouchez A (2018) River biofilm community changes related to pharmaceutical loads emitted by a wastewater treatment plant. Environ Sci Pollut Res 25:9254–9264
Colica G, Li H, Rossi F, Li D, Liu Y, De Philippis R (2014) Microbial secreted exopolysaccharides affect the hydrological behavior of induced biological soil crusts in desert sandy soils. Soil Biol Biochem 68:62–70
D’Abzac P, Bordas F, Van Hullebusch E, Lens PNL, Guibaud G (2010) Effects of extraction procedures on metal binding properties of extracellular polymeric substances (EPS) from anaerobic granular sludges. Colloids Surf B Biointerfaces 80:161–168
Didoné EJ, Minella JPG, Merten GH (2015) Quantifying soil erosion and sediment yield in a catchment in southern Brazil and implications for land conservation. J. Soils Sediments 15:2334–2346
Edwards SJ, Kjellerup BV (2013) Applications of biofilms in bioremediation and biotransformation of persistent organic pollutants, pharmaceuticals/personal care products, and heavy metals. Appl Microbiol Biotechnol 97:9909–9921
EMBRAPA – Empresa Brasileira de Pesquisa Agropecuária (2013) Sistema Brasileiro de Classificação de Solos. 3.ed. Brasília: Embrapa. 353 p. (in Portuguese)
Fechner LC, Versace F, Gourlay-Francé C, Tusseau-Vuillemin M-H (2012) Adaptation of copper community tolerance levels after biofilm transplantation in an urban river. Aquat Toxicol 106–107:32–41
Fernandes G, Aparicio VC, Bastos MC, De Gerónimo E, Labanowski J, Prestes OD, Zanella R, dos Santos DR (2019) Indiscriminate use of glyphosate impregnates river epilithic biofilms in southern Brazil. Sci Total Environ 651:1377–1387
Fialho LL, Silva WTL, Milori DMBP, Simões ML, Matin-Neto L (2010) Characterization of organic matter from composting of different residues by physicochemical and spectroscopic methods. Bioresour Technol 101:1927–1934
Fish KE, Collins R, Green NH, Sharpe RL, Douterelo I, Osborn AM, Boxall JB (2015) Characterization of the physical composition and microbial community structure of biofilms within a model full-scale drinking water distribution system. PLoS One 10:e0115824
Flemming HC, Wingender J (2010) The biofilm matrix. Nat. Rev. Microbiol. 8:623–633
Flemming HC, Neu TR, Wozniak DJ (2007) The EPS matrix: the “house of biofilm cells”. J Bacteriol 189:7945–7947
Jiao Y, Cody GD, Harding AK, Wilmes P, Schrenk M, Wheeler KE, Banfield JF, Thelen MP (2010) Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl Environ Microbiol 76:2916–2922
Julien C, Laurent E, Legube B, Thomassin J-H, Mondamert L, Labanowski J (2014) Investigation on the iron-uptake by natural biofilms. Water Res 50:212–220
Kottek M, Rubel F (2010) Observed and projected climate shifts 1901-2100 depicted by world maps of the Köppen-Geiger climate classification. Meteorol Z 19:135–141
Lambert A-S, Morin S, Artigas J, Volat B, Coquery M, Neyra M, Pesce S (2012) Structural and functional recovery of microbial biofilms after a decrease in copper exposure: influence of the presence of pristine communities. Aquat Toxicol 109:118–126
Lawrence JR, Dynes JJ, Korber DR, Swerhone GDW, Leppard GG, Hitchcock AP (2012) Monitoring the fate of copper nanoparticles in river biofilms using scanning transmission X-ray microscopy (STXM). Chem Geol 329:18–25
Lin H, Ye C, Lv L, Zheng CR, Zhang S, Zheng L, Zhao Y, Yu X (2014) Characterization of extracellular polymeric substances in the biofilms of typical bacteria by the sulfur K-edge XANES spectroscopy. J Environ Sci 26:1763–1768
Matin A, Khan Z, Zaidi SMJ, Boyce MC (2011) Biofouling in reverse osmosis membranes for seawater desalination. Phenomena and prevention Desalination 281:1–16
Morin S, Pesce S, Tlili A, Coste M, Montuelle B (2010) Recovery potential of periphytic communities in a river impacted by a vineyard watershed. Ecol Indic 10:419–426
Ramos RLO, Abrantes FJ (2017) Marau - IBGE Cidades. INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA – IBGE (in Portuguese)
Ratledge C (1991) Microorganisms for lipids. Acta Biotechnol 11:429–438
Reichhardt JAG, Ferreira L-M, Joubert KV, Clemons DA, Stevens LC (2015) Analysis of the Aspergillus fumigatus biofilm extracellular matrix by solid-state nuclear magnetic resonance spectroscopy Courtney. Eukaryot Cell 14:1064–1072
Rheinheimer DS, de Castro Lima JAM, de Vargas JPR et al (2020) Pesticide bioaccumulation in epilithic biofilms as biomarkers of agricultural activities in a representative watershed. Environ Monit Assess 192:6
Ribot M, von Schiller D, Sabater F, Martí E (2015) Biofilm growth and nitrogen uptake responses to increases in nitrate and ammonium availability. Aquat Sci 77:1–13
Rocha LL, Aguiar Cordeiro R, Cavalcante RM, Nascimento RF, Martins SCS, Santaella ST, Melo VMM (2007) Isolation and characterization of phenol-degrading yeasts from an oil refinery wastewater in Brazil. Mycopathologia 164:183–188
Rossel RAV, Behrens T (2010) Using data mining to model and interpret soil diffuse reflectance spectra. Geoderma 158:46–54
Sabater S, Guasch H, Ricart M, Romaní A, Vidal G, Klünder C, Schmitt-Jansen M (2007) Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal Bioanal Chem 387:1425–1434
Sambalova O, Thorwarth K, Heeb NV, Bleiner D, Zhang Y, Borgschulte A, Kroll A (2018) Carboxylate functional groups mediate interaction with silver nanoparticles in biofilm matrix. ACS Omega 3:724–733
Schlafer S, Meyer RL (2017) Confocal microscopy imaging of the biofilm matrix. J. Microbiol Methods 138:50–59
Schneider SC, Lindstrom EA (2011) The periphyton index of trophic status PIT: a new eutrophication metric based on non-diatomaceous benthic algae in Nordic rivers. Hydrobiologia 665:143–155
Silverstein RM, Webster FX, Kiemle DJ (2006) Identificação espectrométrica de compostos orgânicos. LTC, Rio de Janeiro, Brazil (in Potuguese)
Stewart PS, Franklin MJ (2008) Physiological heterogeneity in biofilms. Nat Rev Microbiol 6:199–210
Stewart TJ, Traber J, Kroll A, Behra R, Sigg L (2012) Characterization of extracellular polymeric substances (EPS) from periphyton using liquid chromatography-organic carbon detection–organic nitrogen detection (LC-OCD-OND). Environ Sc Pollut Res 20:3214–3223
Sun XF, Wang SG, Zhang XM, Paul Chen J, Li XM, Gao BY, Ma Y (2009) Spectroscopic study of Zn2+ and Co2+ binding to extracellular polymeric substances (EPS) from aerobic granules. J Colloid Interface Sci 335:11–17
Tank J, Dodds WK (2003) Nutrient limitation of epilithic and epixylic biofilms in ten north American streams. Freshw Biol 48(6):1031–1049
Tiecher T, Minella JPG, Caner L, Evrard O, Zafar M, Capoane V, Le Gall M, Santos DR (2017) Quantifying land use contributions to suspended sediment in a large cultivated catchment of southern Brazil (Guaporé River, Rio Grande do Sul). Agric Ecosyst Environ 237:95–108
Vila-Costa M, Bartrons M, Catalan J, Casamayor EO (2014) Nitrogen-cycling genes in epilithic biofilms of oligotrophic high-altitude lakes (Central Pyrenees, Spain). Microb Ecol 68:60–69
Wuertz S, Okabe S, Hausner M (2004) Microbial communities and their interactions in biofilm systems: an overview. Water Sci Technol 49:327–336
Zhang Y, Wang F, Zhu X, Zeng J, Zhao Q, Jiang X (2015) Extracellular polymeric substances govern the development of biofilm and mass transfer of polycyclic aromatic hydrocarbons for improved biodegradation. Bioresour Technol 193:274–280
Acknowledgments
The authors thank Clarissa Piccinin Frizzo for assistance with spectral measurements and Núcleo de Análises e Pesquisas Orgânicas of the Chemistry Department of the Universidade Federal de Santa Maria for technical support.
Funding
This research was funded by Mais Água of FINEP/FEPAGRO, National Council for Scientific and Technological Development (CNPq, Brazil) for fellowships awarded to DS Rheinheimer (309515/2015–7), FAPERGS (19/2551–0001641-7), and Région Nouvelle Aquitaine (France).
Author information
Authors and Affiliations
Contributions
GF, MCB, JL, and DRS participated in the conception of the work. GF, MCB, LM, RAB, and DRS participated in methodology, data acquisition, and data interpretation. GF leads the writing of the manuscript. GF, DRS, MCB, and LM purchased funding. All authors contributed to the manuscript correction, discussion, and interpretation of results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible editor: Gerald Thouand
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernandes, G., Bastos, M.C., Mondamert, L. et al. Organic composition of epilithic biofilms from agricultural and urban watershed in South Brazil. Environ Sci Pollut Res 28, 28808–28824 (2021). https://doi.org/10.1007/s11356-020-11389-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11389-5