Abstract
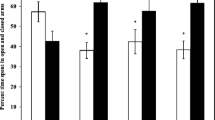
Ecotoxicological studies are necessary in order to evaluate the effects of environmental exposure of chemicals on wild animals and their ecological consequences. Particularly, neurobehavioral effects of heavy metal elements on wild rodents have been scarcely investigated. In the present study, we analyzed the effect of metal bioaccumulation (Pb, As, Mg, Ni, and Zn) in the brain and in the liver on exploratory activity, learning, memory, and on some dopaminergic markers in the wild rodent Liomys irroratus living inside mine tailings, at Huautla, Morelos, Mexico. We found higher Pb concentration but lower Zn in striatum, nucleus accumbens, midbrain, and hippocampus in exposed animals in comparison to rodents from the reference site. Exposed rodents exhibited anxious behavior evaluated in the open field, while no alterations in learning were found. However, they displayed slight changes in the memory test in comparison to reference group. The neurochemical evaluation showed higher levels of dopamine and 5-hydroxyindolacetic acid in midbrain, while lower levels of metabolites dihydroxyphenyl acetic acid and homovanillic acid in striatum of exposed rodents. In addition, mRNA expression levels of dopaminergic D2 receptors in nucleus accumbens were lower in animals from the mining zone than in animals from the reference zone. This is the first study that shows that chronic environmental exposure to metals results in behavioral and neurochemical alterations in the wild rodent L. irroratus, a fact that may comprise the survival of the individuals resulting in long-term effects at the population level. Finally, we suggest the use of L. irroratus as a sentinel species for environmental biomonitoring of mining sites.




Similar content being viewed by others
References
Abou-Arab AAK (2001) Heavy metal contents in Egyptian meat and the role of detergent washing on their levels. Food Chem Toxicol 39(6):593–599. https://doi.org/10.1016/S0278-6915(00)00176-9
Al Sayegh Petkovšek S, Kopušar N, Kryštufek B (2014) Small mammals as biomonitors of metal pollution: a case study in Slovenia. Environ Monit Assess 186:4261–4274. https://doi.org/10.1007/s10661-014-3696-7
Alcaro A, Huber R, Panksepp J (2007) Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev 56:283–321. https://doi.org/10.1016/j.brainresrev.2007.07.014
Antonio MT, López N, Leret ML (2002) Pb and Cd poisoning during development alters cerebellar and striatal function in rats. Toxicology 176:59–66. https://doi.org/10.1016/S0300-483X(02)00137-3
Bahi A, Dreyer JL (2019) Dopamine transporter (DAT) knockdown in the nucleus accumbens improves anxiety- and depression-related behaviors in adult mice. Behav Brain Res 359:104–115. https://doi.org/10.1016/j.bbr.2018.10.028 Epub 2018 Oct 24
Bailey KR, Crawley JN (2009) Anxiety-related behaviors in mice. In: Buccafusco J (ed) Methods of behavior analysis in neuroscience, 2nd edn. CRC Press - Frontiers in Neuroscience, United of States of America, pp 77–101
Baldo BA, Sadeghian K, Basso AM, Kelley AE (2002) Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res 137:165–177. https://doi.org/10.1016/S0166-4328(02)00293-0
Bardullas U, Limón-Pacheco JH, Giordano M, Carrizales L, Mendoza-Trejo MS, Rodríguez VM (2009) Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharmacol 239(2):169–177. https://doi.org/10.1016/j.taap.2008.12.004 Epub 2008 Dec 14
Bardullas U, Giordano M, Rodríguez VM (2011) Chronic atrazine exposure causes disruptions of the locomotor activity and alters the striatal dopaminergic system of the male Sprague-Dawley rat. Neurotoxicol Teratol 33(2):263–272. https://doi.org/10.1016/j.ntt.2010.09.001 Epub 2010 Sep 16
Bardullas U, Giordano M, Rodríguez VM (2013) Atrazine is primarily responsible for the toxicity of long-term exposure to a combination of atrazine and inorganic arsenic in the nigrostriatal system of the albino rat. Neurotoxicol Teratol 40:59–66. https://doi.org/10.1016/j.ntt.2013.10.003 Epub 2013 Oct 24
Basha MR, Wei W, Brydie M, Razmiafshari M, Zawia NH (2003) Lead-induced developmental perturbations in hippocampal Sp1 DNA-binding are prevented by zinc supplementation: in vivo evidence for Pb and Zn competition. Int J Dev Neurosci 21:1–12. https://doi.org/10.1016/S0736-5748(02)00137-5
Basu N, Scheuhammer A, Grochowina N, Klenavic K, Evans D, O'Brien M, Chan HM (2005a) Effects of mercury on neurochemical receptor-binding characteristics in wild mink. Environ Toxicol Chem 24:1444–1450. https://doi.org/10.1021/es0483746
Basu N, Stamler CJ, Loua KM, Chan HM (2005b) An interspecies comparison of mercury inhibition on muscarinic acetylcholine receptor binding in the cerebral cortex and cerebellum. Toxicol Appl Pharmacol 205:71–76. https://doi.org/10.1016/j.taap.2004.09.009
Basu N, Scheuhammer AM, Bursian SJ, Elliott J, Rouvinen-Watt K, Chan HM (2007a) Mink as a sentinel species in environmental health. Environ Res 103:130–144. https://doi.org/10.1016/j.envres.2006.04.005
Basu N, Scheuhammer AM, Evans RD, O'Brien M, Chan HM (2007b) Cholinesterase and monoamine oxidase activity in relation to mercury levels in the cerebral cortex of wild river otters. Hum Exp Toxicol 26:213–220. https://doi.org/10.1177/0960327107070570
Basu N, Scheuhammer AM, Rouvinen-Watt K, Grochowina N, Evans RD, O’Brien M, Chan HM (2007c) Decreased N-methyl-d-aspartic acid (NMDA) receptor levels are associated with mercury exposure in wild and captive mink. NeuroToxicology 28:587–593. https://doi.org/10.1016/j.neuro.2006.12.007
Basu N, Scheuhammer AM, Rouvinen-Watt K, Evans RD, Grochowina N, Chan LH (2008) The effects of mercury on muscarinic cholinergic receptor subtypes (M1 and M2) in captive mink. NeuroToxicology 29:328–334. https://doi.org/10.1016/j.neuro.2008.01.003
Basu N, Scheuhammer AM, Sonne C, Letcher RJ, Born EW, Dietz R (2009) Is dietary mercury of neurotoxicological concern to wild polar bears (Ursus maritimus)? Environ Toxicol Chem 28:133–140. https://doi.org/10.1897/08-251.1
Betharia S, Maher TJ (2012) Neurobehavioral effects of lead and manganese individually and in combination in developmentally exposed rats. NeuroToxicology 33:1117–1127. https://doi.org/10.1016/j.neuro.2012.06.002
Bortey-Sam N, Nakayama SM, Ikenaka Y, Akoto O, Baidoo E, Yohannes YB, Ishizuka M (2015) Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: estimation of the daily intakes and target hazard quotients (THQs). Ecotox Environ Saf 111:160–167
Boschen SL, Wietzikoski EC, Winn P, Cunha CD (2011) The role of nucleus accumbens and dorsolateral striatal D2 receptors in active avoidance conditioning. Neurobiol Learn Mem 96:254–262. https://doi.org/10.1016/j.nlm.2011.05.002
Bridges CC, Zalups RK (2005) Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol 204:274–308. https://doi.org/10.1016/j.taap.2004.09.007
Cadena-Salgado M (2003) Efectos de la perturbación y estacionalidad en la comunidad de roedores en una selva baja caducifolia en Morelos, México. Universidad de las Americas, Puebla
Cao X, Huang S, Ruan D (2008) Enriched environment restores impaired hippocampal long-term potentiation and water maze performance induced by developmental lead exposure in rats. Dev Psychobiol 50:307–313. https://doi.org/10.1002/dev.20287
Castro-Arellano I, Timm R, Álvarez-Castañeda S (2016) Heteromys irroratus. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2016-3.RLTS.T12074A22225187.en Copyright:
Cid N, Ibáñez C, Palanques A, Prat N (2010) Patterns of metal bioaccumulation in two filter-feeding macroinvertebrates: exposure distribution, inter-species differences and variability across developmental stages. Sci Total Environ 408(14):2795–2806
Coban A, Filipov NM (2007) Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J Neurochem 100(5):1177–1187
Cory-Slechta D, O’Mara D, Brockel B (1998) Nucleus accumbens dopaminergic medication of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J Pharmacol Exp Ther 286:794–805
Dornbos P, Strom S, Basu N (2013) Mercury exposure and neurochemical biomarkers in multiple brain regions of Wisconsin River Otters (Lontra canadensis). Ecotoxicology 22:469–475. https://doi.org/10.1007/s10646-013-1040-6
De la Cruz Guarneros N (2018) Efecto de la bioacumulación de metales en la población de Liomys irroratus (Gray 1968) que habita los jales de Huautla, Morelos: Un enfoque multimarcadores. Autonomous University of Morelos State, Mexico
Dowler RC, Genoways HH (1978) Liomys irrotarus. Mamm Species 82:1–6
Duarte-Guterman P, Yagi S, Chow C, Galea LAM (2015) Hippocampal learning, memory, and neurogenesis: effects of sex and estrogens across the lifespan in adults. Horm Behav 74:37–52. https://doi.org/10.1016/j.yhbeh.2015.05.024
Eeva T, Ahola M, Lehikoinen E (2009) Breeding performance of blue tits (Cyanistes caeruleus) and great tits (Parus major) in a heavy metal polluted area. Environ Pollut 157:3126–3131. https://doi.org/10.1016/j.envpol.2009.05.040
Erry BV, Macnair MR, Meharg AA, Shore RF (1999) Seasonal variation in dietary and body organ arsenic concentrations in wood mice Apodemus sylvaticus and bank voles Clethrionomys glareolus. Bull Environ Contam Toxicol 63:567–574. https://doi.org/10.1007/s001289901018
Erry BV, Macnair MR, Meharg AA, Shore RF (2005) The distribution of arsenic in the body tissues of wood mice and bank voles. Arch Environ Contam Toxicol 49:569–576. https://doi.org/10.1007/s00244-004-0229-3
Espinosa LA, Chavez-Tapia C (2005) Liomys irroratus. In: Ceballos G, Oliva G (eds) Los mamíferos silvestres de México, 1st edn. Comisión nacional para el conocimiento y uso de la biodiversidad- Fondo de Cultura Económica, México, D.F, pp 628–629
Fisone G, Hakansson K, Borgkvist A, Santini E (2007) Signaling in the basal ganglia: postsynaptic and presynaptic mechanisms. Physiol Behav 92:8–14
Flores-Montoya MG, Sobin C (2015) Early chronic lead exposure reduces exploratory activity in young C57BL/6J mice. J Appl Toxicol 35:759–765. https://doi.org/10.1002/jat.3064
Fritsch C, Cosson RP, Cœurdassier M, Raoul F, Giraudoux P, Crini N, Scheifler R (2010) Responses of wild small mammals to a pollution gradient: host factors influence metal and metallothionein levels. Environ Pollut 158(3):827–840
Gall JE, Boyd RS, Rajakaruna N (2015) Transfer of heavy metals through terrestrial food webs: a review. Environ Monit Assess 187(4):201. https://doi.org/10.1007/s10661-015-4436-3
Gedeon Y, Ramesh GT, Wellman PJ, Jadhav AL (2001) Changes in mesocorticolimbic dopamine and D1/D2 receptor levels after low level lead exposure: a time course study. Toxicol Lett 123:217–226. https://doi.org/10.1016/S0378-4274(01)00408-8
Gentsch C, Lichtsteiner M, Feer H (1987) Open field and elevated plus-maze: a behavioural comparison between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats and the effects of chlordiazepoxide. Behav Brain Res 25:101–107. https://doi.org/10.1016/0166-4328(87)90003-9
Gonçalves JT, Schafer ST, Gage FH (2016) Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell 167:897–914. https://doi.org/10.1016/j.cell.2016.10.021
Gray JE (1868) Synopsis of the species of Saccomyinae, or pouched mice, in the collection of the British museum. Proc Zool Soc London 199–206
Guilarte TR, Toscano CD, McGlothan JL, Weaver SA (2003) Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Ann Neurol 53:50–56. https://doi.org/10.1002/ana.10399
Gupta R, Shukla RK, Pandey A, Sharma T, Dhuriya YK, Srivastava P, Singh MP, Siddiqi MI, Pant AB, Khanna VK (2018) Involvement of PKA/DARPP-32/PP1α and β-Arrestin/Akt/GSK-3β signaling in cadmium-induced DA-D2 receptor-mediated motor dysfunctions: protective role of Quercetin. Sci Rep 8:1–18. https://doi.org/10.1038/s41598-018-20342-z
Hartwig A, Schlepegrell R, Beyersmann D (1990) Indirect mechanism of lead-induced genotoxicity in cultured mammalian cells. Mutat Res Genet Toxicol 241:75–82. https://doi.org/10.1016/0165-1218(90)90110-N
He M-D, Xu S-C, Zhang X, Wang Y, Xiong JC, Zhang X, Lu YH, Zhang L, Yu ZP, Zhou Z (2013) Disturbance of aerobic metabolism accompanies neurobehavioral changes induced by nickel in mice. NeuroToxicology 38:9–16. https://doi.org/10.1016/j.neuro.2013.05.011
Hellou J (2011) Behavioural ecotoxicology, an “early warning” signal to assess environmental quality. Environ Sci Pollut Res 18:1–11. https://doi.org/10.1007/s11356-010-0367-2
Hernández-Plata I, Giordano M, Díaz-Muñoz M, Rodríguez VM (2015) The herbicide glyphosate causes behavioral changes and alterations in dopaminergic markers in male Sprague-Dawley rat. NeuroToxicology 46:79–91. https://doi.org/10.1016/j.neuro.2014.12.001
Hesel DR (1990) Less than obvious: statistical treatment of data below the detection limit. Environ Sci Technol 39:419–423
Humphries MD, Prescott TJ (2010) The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol 90:385–417. https://doi.org/10.1016/j.pneurobio.2009.11.003
Ijomone OM, Okori SO, Ijomone OK, Ebokaiwe AP (2018) Sub-acute nickel exposure impairs behavior, alters neuronal microarchitecture, and induces oxidative stress in rats’ brain. Drug Chem Toxicol 41:377–384. https://doi.org/10.1080/01480545.2018.1437173
Janssens E, Dauwe T, Pinxten R, Eens M (2003a) Breeding performance of great tits (Parus major) along a gradient of heavy metal pollution. EnvironToxicol Chem 22:1140–1145. https://doi.org/10.1897/1551-5028(2003)022<1140:BPOGTP>2.0.CO;2
Janssens E, Dauwe T, Van Duyse E et al (2003b) Effects of heavy metal exposure on aggressive behavior in a small territorial songbird. Arch Environ Contam Toxicol 45:121–127. https://doi.org/10.1007/s00244-002-0133-7
Johansson L, Pellicciari CE (1988) Lead-induced changes in the stabilization of the mouse sperm chromatin. Toxicology 51:11–24. https://doi.org/10.1016/0300-483X(88)90076-5
Karri V, Schuhmacher M, Kumar V (2016) Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: a general review of metal mixture mechanism in brain. Environ Toxicol Pharmacol 48:203–213. https://doi.org/10.1016/j.etap.2016.09.016
Kasten-Jolly J, Pabello N, Bolivar VJ, Lawrence DA (2012) Developmental lead effects on behavior and brain gene expression in male and female BALB/cAnNTac mice. NeuroToxicology 33:1005–1020. https://doi.org/10.1016/j.neuro.2012.04.017
Kern C, Stanwood G, Smith DR (2010) Pre-weaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse 64:363–378. https://doi.org/10.1002/syn.20736
Krey A, Kwan M, Chan HM (2014) In vivo and in vitro changes in neurochemical parameters related to mercury concentrations from specific brain regions of polar bears (Ursus maritimus). Environ Toxicol Chem 33:2463–2471. https://doi.org/10.1002/etc.2685
Krey A, Ostertag SK, Chan HM (2015) Assessment of neurotoxic effects of mercury in beluga whales (Delphinapterus leucas), ringed seals (Pusa hispida), and polar bears (Ursus maritimus) from the Canadian Arctic. Sci Total Environ 509:237–247. https://doi.org/10.1016/j.scitotenv.2014.05.134
Kuhlmann AC, McGlothan JL, Guilarte TR (1997) Developmental lead exposure causes spatial learning deficits in adult rats. Neurosci Lett 233:101–104. https://doi.org/10.1016/S0304-3940(97)00633-2
Levengood JM, Heske EJ (2008) Heavy metal exposure, reproductive activity, and demographic patterns in white-footed mice (Peromyscus leucopus) inhabiting a contaminated floodplain wetland. Sci Total Environ 389(2-3):320–328. https://doi.org/10.1016/j.scitotenv.2007.08.050
Lindvall O, Björklund A (1978) Anatomy of the dopaminergic neuron systems in the rat brain. Adv Biochem Psychopharmacol 19:1–23
Liu J, Chen H, Miller DS, Saavedra JE, Keefer LK, Johnson DR, Klaassen CD, Waalkes MP (2001) Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol Pharmacol 60(2):302–309
Lucchi L, Memo M, Airaghi ML, Spano PF, Trabucchi M (1981) Chronic lead treatment induces in rat a specific and differential effect on dopamine receptors in different brain areas. Brain Res 213:397–404. https://doi.org/10.1016/0006-8993(81)90244-4
Ma T, Chen HH, Ho IK (1999) Effects of chronic lead (Pb) exposure on neurobehavioral function and dopaminergic neurotransmitter receptors in rats. Toxicol Lett 105:111–121. https://doi.org/10.1016/S0378-4274(98)00388-9
Maceda-Veiga A, Monroy M, de Sostoa A (2012) Metal bioaccumulation in the Mediterranean barbel (Barbus meridionalis) in a Mediterranean River receiving effluents from urban and industrial wastewater treatment plants. Ecotox Environ Saf 76:93–101
Márquez-Ferrando R, Santos X, Pleguezuelos JM, Ontiveros D (2009) Bioaccumulation of heavy metals in the lizard Psammodromus algirus after a tailing-dam collapse in Aznalcóllar (Southwest Spain). Arch Environ Contam Toxicol 56(2):276–285
Meador JP, Ernest D, Hohn AA, Tilbury K, Gorzelany J, Worthy G, Stein JE (1999) Comparison of elements in bottlenose dolphins stranded on the beaches of Texas and Florida in the Gulf of Mexico over a one-year period. Arch Environ Contam Toxicol 36:87–98. https://doi.org/10.1007/s002449900446
Moreira EG, Vassilieff I, Vassilieff VS (2001) Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol 23:489–495. https://doi.org/10.1016/S0892-0362(01)00159-3
Mussali P, Rojas E, Mussali-Galante P et al (2014) Genetic structure and diversity of animal populations exposed to metal pollution. Rev Environ Contam Toxicol:227. https://doi.org/10.1007/978-3-319-01327-5
Mussali-Galante P, Tovar-Sánchez E, Valverde M, Valencia-Cuevas L, Rojas E (2013) Evidence of population genetic effects in Peromyscus melanophrys chronically exposed to mine tailings in Morelos, Mexico. Environ Sci Pollut Res 20:7666–7679. https://doi.org/10.1007/s11356-012-1263-8
Nam DH, Yates D, Ardapple P, Evers DC, Schmerfeld J, Basu N (2012) Elevated mercury exposure and neurochemical alterations in little brown bats (Myotis lucifugus) from a site with historical mercury contamination. Ecotoxicology 21:1094–1101. https://doi.org/10.1007/s10646-012-0864-9
Nation JR, Frye GD, Von Stultz J, Bratton GR (1989) Effects of combined lead and cadmium exposure: changes in schedule-controlled responding and in dopamine, serotonin, and their metabolites. Behav Neurosci 103:1108–1114. https://doi.org/10.1037/0735-7044.103.5.1108
Nation JR, Grover CA, Bratton GR, Salinas JA (1990) Behavioral antagonism between lead and cadmium. Neurotoxicol Teratol 12:99–104. https://doi.org/10.1016/0892-0362(90)90119-W
Noelker C, Morel L, Lescot T, Osterloh A, Alvarez-Fischer D, Breloer M, Henze C, Depboylu C, Skrzydelski D, Michel PP, Dodel RC, Lu L, Hirsch EC, Hunot S, Hartmann A (2013) Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep 3:1393. https://doi.org/10.1038/srep01393
Ostertag SK, Stern GA, Wang F, Lemes M, Chan HM (2013) Mercury distribution and speciation in different brain regions of beluga whales (Delphinapterus leucas). Sci Total Environ 456:278–286. https://doi.org/10.1016/j.scitotenv.2013.03.106
Ostertag SK, Shaw AC, Basu N, Chan HM (2014) Molecular and neurochemical biomarkers in arctic beluga whales (Delphinapterus leucas) were correlated to brain mercury and selenium concentrations. Environ Sci Technol 48:11551–11559. https://doi.org/10.1021/es501369b
Paxinos G, Watson C (2007) The rat brain, 6th edn. Elsevier
Pennartz CMA, Groenewegen HJ, Lopes Da Silva FH (1994) The nucleus accumbens as a complex of functionally distinct neuronal ensemples: an integration of behavioral, electrophysiological and anatomical data. Prog Neurobiol 42:719–761
Peres TV, Schettinger MRC, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M (2016) Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol:17. https://doi.org/10.1186/s40360-016-0099-0
Pérez-Rial S, García-Gutiérrez MS, Molina JA, Pérez-Nievas BG, Ledent C, Leiva C, Leza JC, Manzanares J (2011) Increased vulnerability to 6-hydroxydopamine lesion and reduced development of dyskinesias in mice lacking CB1 cannabinoid receptors. Neurobiol Aging 32(4):631–645. https://doi.org/10.1016/j.neurobiolaging.2009.03.017 Epub 2009 May 5
Petering HG (1978) Some observations on the interaction of zinc, copper, and iron metabolism in lead and cadmium toxicity. Environ Health Perspect 25:141–145
Pokora MJ, Richfield EK, Cory-Slechta DA (1996) Preferential vulnerability of nucleus accumbens dopamine binding sites to low-level lead exposure: time course of effects and interactions with chronic dopamine agonist treatments. J Neurochem 67:1540–1550. https://doi.org/10.1046/j.1471-4159.1996.67041540.x
Rai A, Maurya SK, Khare P, Srivastava A, Bandyopadhyay S (2010) Characterization of developmental neurotoxicity of As, Cd, and Pb mixture: synergistic action of metal mixture in glial and neuronal functions. Toxicol Sci 118:586–601. https://doi.org/10.1093/toxsci/kfq266
Ramesh GT, Jadhav AL (1998) Region-specific alterations in tyrosine hydroxylase activity in rats exposed to lead. Mol Cell Biochem 189:19–24. https://doi.org/10.1023/a:1006896111280
Reddy GR, Zawia NH (2000) Lead exposure alters Egr-1 DNA-binding in the neonatal rat brain. Int J Dev Neurosci 18:791–795. https://doi.org/10.1016/S0736-5748(00)00048-4
Reiter LW, Anderson GE, Laskey JW, Cahill DF (1975) Developmental and behavioral changes in the rat during chronic exposure to lead. Environ Health Perspect 12:119–123
Rodríguez VM, Dufour L, Carrizales L, Díaz-Barriga F, Jiménez-Capdeville ME (1998) Effects of oral exposure to mining waste on in vivo dopamine release from rat striatum. Environ Health Perspect 106:487–491. https://doi.org/10.1289/ehp.98106487
Rodríguez VM, Limón-Pacheco JH, Mendoza-Trejo MS, González-Gallardo A, Hernández-Plata I, Giordano M (2013) Repeated exposure to the herbicide atrazine alters locomotor activity and the nigrostriatal dopaminergic system of the albino rat. NeuroToxicology 34:82–94. https://doi.org/10.1016/j.neuro.2012.10.012
Rodríguez VM, Limón-Pacheco JH, Del Razo LM, Giordano M (2016) Effects of inorganic arsenic exposure on glucose transporters and insulin receptor in the hippocampus of C57BL/6 male mice. Neurotoxicol Teratol 54:68–77. https://doi.org/10.1016/j.ntt.2016.02.001
Sánchez-Chardi A, Peñarroja-Matutano C, Ribeiro CAO, Nadal J (2007) Bioaccumulation of metals and effects of a landfill in small mammals. Part II. The wood mouse, Apodemus sylvaticus. Chemosphere 70:101–109. https://doi.org/10.1016/j.chemosphere.2007.06.047
Sánchez-Chardi A, Peñarroja-Matutano C, Borrás M, Nadal J (2009) Bioaccumulation of metals and effects of a landfill in small mammals Part III: structural alterations. Environ Res 109:960–967. https://doi.org/10.1016/j.envres.2009.08.004
Sanders T, Liu Y, Buchner V, Tchounwou PB (2009) Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health 24:15–45
Santos-Moreno A, Santiago-Marcial AE (2012) Área de actividad y movimientos de Liomys irroratus (Rodentia: Heteromyidae) en una selva mediana de Tuxtepec, Oaxaca, México. Revista Mexicana de Biodiversidad 83:496–502
Schneider JS, Lee MH, Anderson DW, Zuck L, Lidsky TI (2001) Enriched environment during development is protective against lead-induced neurotoxicity. Brain Res 896:48–55. https://doi.org/10.1016/S0006-8993(00)03249-2
Sharma RP, Shupe JL (1977) Lead, cadmium, and arsenic residues in animal tissues in relation to those in their surrounding habitat. Sci Total Environ 7:53–62. https://doi.org/10.1016/0048-9697(77)90016-X
Sharma S, Rakoczy S, Brown-Borg H (2010) Assessment of spatial memory in mice. Life Sci 87:521–536
Sobolewski M, Varma G, Adams B, Anderson DW, Schneider JS, Cory-Slechta DA (2018) Developmental lead exposure and prenatal stress result in sex-specific reprograming of adult stress physiology and epigenetic profiles in brain. Toxicol Sci 163:478–489. https://doi.org/10.1093/toxsci/kfy046
Solis Miranda BM (2016) Aislamiento de bacterias de jales mineros y análisis de su potencial para la remediación de sitios contaminados con metales pesados. Universidad Autónoma del Estado de Morelos
Song X, Fiati Kenston SS, Kong L, Zhao J (2017) Molecular mechanisms of nickel induced neurotoxicity and chemoprevention. Toxicology 392:47–54. https://doi.org/10.1016/j.tox.2017.10.006
Stone D, Jepson P, Kramarz P, Laskowski R (2001) Time to death response in carabid beetles exposed to multiple stressors along a gradient of heavy metal pollution. Environ Pollut 113:239–244. https://doi.org/10.1016/S0269-7491(00)00134-2
Svensson L, Ahlenius S (1983) Suppression of exploratory locomotor activity by the local application of dopamine or l-Noradrenaline to the nucleus accumbens of the rat. Pharmacol Biochem Behav 19:693–699
Takeda A (2000) Movement of zinc and its functional significance in the brain. Brain Res Rev 34:137–148. https://doi.org/10.1016/S0165-0173(00)00044-8
Takeda A, Tamano H, Tochigi M, Oku N (2005) Zinc homeostasis in the hippocampus of zinc-deficient young adult rats. Neurochem Int 46:221–225. https://doi.org/10.1016/j.neuint.2004.10.003
Takeda A, Tamano H, Kan F, Itoh H, Oku N (2007) Anxiety-like behavior of young rats after 2-week zinc deprivation. Behav Brain Res 177:1–6. https://doi.org/10.1016/j.bbr.2006.11.023
Tarale P, Chakrabarti T, Sivanesan S et al (2016) Potential role of epigenetic mechanism in manganese induced neurotoxicity. Biomed Res Int:1–18. https://doi.org/10.1155/2016/2548792
Terry A (2009) Spatial navigation (water maze) tasks. In: Buccafusco J (ed) Methods of behavior analysis in neuroscience, 2nd edn. CRC Press - Frontiers in Neuroscience, States of America, pp 267–280
Tête N, Durfort M, Rieffel D, Scheifler R, Sánchez-Chardi A (2014) Histopathology related to cadmium and lead bioaccumulation in chronically exposed wood mice, Apodemus sylvaticus, around a former smelter. Sci Total Environ 481:167–177. https://doi.org/10.1016/j.scitotenv.2014.02.029
Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA (2000) The nigrostriatal dopaminergic system as a preferential target of repeated exposure to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci 20(24):9207–9214
Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, Cory-Slechta DA (2003) Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson’s disease phenotype. Eur J Neurosci 18(3):589–600
Tovar-Sánchez E, Cervantes LT, Martínez C, Rojas E, Valverde M, Ortiz-Hernández ML, Mussali-Galante P (2012) Comparison of two wild rodent species as sentinels of environmental contamination by mine tailings. Environ Sci Pollut Res 19:1677–1686. https://doi.org/10.1007/s11356-011-0680-4
Tovar-Sánchez E, Mussali-Galante P, Martínez-Pacheco M et al (2016) Relationship between genotoxic damage and arsenic blood concentrations in individuals residing in an arsenic contaminated area in Morelos, México. Rev Int Contam Ambient 32:101–117
Verina T, Rohde CA, Guilarte TR (2007) Environmental lead exposure during early life alters granule cell neurogenesis and morphology in the hippocampus of young adult rats. Neuroscience 145:1037–1047. https://doi.org/10.1016/j.neuroscience.2006.12.040
Volke S, Velasco T, De la Rosa P, Solórzano O (2004) Evaluación de tecnologías de remediación para suelos contaminados con metales. Etapa I. Secretaría de Medio Ambiente y Recursos Naturales, Mexico
Volke S, Velasco T, De la Rosa P, Solórzano O (2005) Evaluación de tecnologías de remediación para suelos contaminados con metales.Etapa II. Secretaría de Medio Ambiente y Recursos Naturales, Mexico
Webster RA (2001) Dopamine. In: Webster R (ed) Neurotransmitters, drugs and brain function. Wiley, United States of America, pp 137–161
Widzowski DV, Cory-Slechta DA (1994) Homogeneity of regional brain lead concentrations. NeuroToxicology 15:295–307
Widzowski DV, Finkelstein JN, Pokora MJ, Cory-Slechta DA (1994) Time course of postnatal lead-induced changes in dopamine receptors and their relationship to changes in dopamine sensitivity. NeuroToxicology 15:853–865
Zawia NH, Crumpton T, Brydie M, Reddy GR, Razmiafshari M (2000) Disruption of the zinc finger domain: a common target that underlies many of the effects of lead. NeuroToxicology 21:1069–1080
Acknowledgments
The authors would like to thank Dr. Evangelina Delgado-González and Dr. Rocio Brenda Anguiano Serrano, Evodio Rendón Alquicira, Lucas Lucio Arredondo Ortega, Amado García Sánchez, Miguel Santoyo Martínez, Juan Ramírez Zamora, Carlos Ángel Quintana Ocampo, Carlos Vergara Allende, Joel Daniel Castañeda Espinosa, Fidel Ocampo Bautista, Natalia De la Cruz Guarneros, Janet Esteves Aguilar, Marcos Rosas Ramírez, Miguel Ángel Galván Ramírez, Anaid Fuentes Reza, and Dania Rebolledo Salinas for their technical assistance. Dr. Isela Hernández Plata was a CONACyT posdoctoral fellow (fellowship 164300) at the Universidad Autónoma del Estado de Morelos. This research was supported by CONACYT grant # 240414 to P. Mussali-Galante and #251510 to V.M. Rodríguez.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Hernández-Plata, I., Rodríguez, V.M., Tovar-Sánchez, E. et al. Metal brain bioaccumulation and neurobehavioral effects on the wild rodent Liomys irroratus inhabiting mine tailing areas. Environ Sci Pollut Res 27, 36330–36349 (2020). https://doi.org/10.1007/s11356-020-09451-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09451-3