Abstract
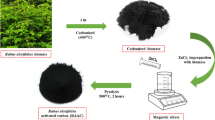
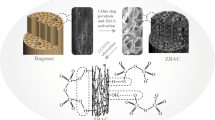
In this work, zirconium chloride octahydrate/CTAB/vetiver grass-activated carbon (ZR/CTAB/VGAC) was prepared from vetiver grass-activated carbon (VGAC), using zirconium chloride octahydrate (ZR) and cetyltrimethylammonium bromide (CTAB) as modifiers. The optimized conditions of the simultaneous phosphate and nitrate removal by ZR/CTAB/VGAC were discussed, including amount of adsorbent, initial concentration, pH, contact time, and temperature. The simultaneous removal efficiency of phosphate and nitrate was 96.50% and 51.17% under optimized conditions. The structural and morphology of ZR/CTAB/VGAC was investigated by using automatic volumetric adsorption analyzer (BET), scanning electron microscopy (SEM), energy dispersive x-ray analysis (EDAX), and Fourier transform infrared spectroscopy (FTIR). It was found that the removal efficiencies of phosphate and nitrate were enhanced dramatically because ZR and CTAB were introduced on the surface of VGAC after modification. Moreover, the adsorption data fitted significantly well with Freundlich isotherm model. It was described well by pseudo-second-order kinetic model. Phosphate and nitrate adsorbed via chemisorption (ion exchange) by ZR, CTAB, and functional groups of the surface of ZR/CTAB/VGAC. Electrostatic adsorption of AC in ZR/CTAB/VGAC also played an important role in the adsorption process. ZR/CTAB/VGAC is an excellent adsorbent, which could be applied to remove nitrate and phosphate from wastewater.










Similar content being viewed by others
References
Alagumuthu G, Rajan M (2010) Equilibrium and kinetics of adsorption of fluoride onto zirconium impregnated cashew nut shell carbon. Chem Eng J 158(3):451–457
Chen YY, Lu S, Hui HX (2017) Remediation effect and action pathway of Indian mustard and vetiveria on Pb contaminated soil. J Environ Sci 30(9):1365–1372
Du C, Xue Y, Wu Z, Wu Z (2017) Microwave-assisted one-step preparation of macadamia nut shell-based activated carbon for efficient adsorption of Reactive Blue. New J Chem 41(24):15373–15383
Jibril M, Noor SN, Muhammad AZZ (2015) Adsorption of benzene and toluene onto KOH activated coconut shell based carbon treated with NH3. Int Biodeterior Biodegradation 102:245–255
Kilpimaa S, Runtti H, Kangas T (2014) Removal of phosphate and nitrate over a modified carbon residue from biomass gasification. Chem Eng Res Des 92(10):1923–1933
Lada M, Seelawut D (2018) Low cost and easy rice husk modification to efficiently enhance ammonium and nitrate adsorption. Int J Recycl Organ Waste Agric:s40093-018-0200-3
Laksaci H, Khelifi A, Trari M (2017) Synthesis and characterization of microporous activated carbon from coffee grounds using potassium hydroxides. J Clean Prod 147(Complete):254–262
Li HF (2018) Experimental study on adsorption of nitrogen and phosphorus from wastewater by modified zeolite. Dissertation, Hebei university of engineering
Li GZ, Li JX, Tan W (2016) Preparation and characterization of the hydrogen storage activated carbon from coffee shell by microwave irradiation and KOH activation. Int Biodeterior Biodegradation:S0964830516301512
Li C, Yao J, Zhang TC (2017a) Simultaneous removal of nitrogen and phosphorus by cetylpyridinium bromide modified zeolite. Water Sci Technol:wst2017459
Li SJ, Han KH, Li JX (2017b) Preparation and characterization of super activated carbon produced from gulfweed by KOH activation. Microporous Mesoporous Mater 243:291–300
Lianggen A, Fan X, Yang R (2019) Enhanced nitrate removal by micro-electrolysis using Fe0 and surfactant modified activated carbon. Chem Eng J
Lin JW, Zhan YH, Lu X (2012) Adsorption characteristics of zeolite modified by zirconium on phosphate and ammonium in water. China Environ Sci 32(11):2023–2031
Lin B, Yang XM, Li LN (2017) Research on nitrogen and phosphorus adsorption efficiency of carbonized straw on urban sewage. Anhui Agri Sci Bull 14:86–88
Liu JY, Wan LH, Zhang L (2011) Effect of pH, ionic strength, and temperature on the phosphate adsorption onto lanthanum-doped activated carbon fiber. J Colloid Interface Sci 364(2):490–496
Liu Q, Hu P, Wang J (2015) Phosphate adsorption from aqueous solutions by zirconium (IV) loaded cross-linked chitosan particles. J Taiwan Inst Chem Eng:59
Liu P, Wang HB, Li GZ (2017) Optimization of activated carbon activation conditions based on response surface method. Biomass Chem Eng 2
Liu Y, Liu X, Zhang G (2018) Adsorptive removal of sulfamethazine and sulfamethoxazole from aqueous solution by hexadecyl trimethyl ammonium bromide modified activated carbon. Colloids Surf A Physicochem Eng Asp:12.041
MAO WJ, Zou S, Tang T (2018) Preparation and adsorption properties of activated carbon from walnut shell. Chem Eng Manag 34:94–95
Mazarji M, Aminzadeh (2017) Removal of nitrate from aqueous solution using modified granular activated carbon. J Mol Liq 233:139–148
Mou RZ, Liu H (2010) Application of printing and dyeing wastewater in seawater coagulation enhanced phosphorus removal technology. Water Supply Drain 12:58–60
Parlayıcı Ş, Pehlivan E (2017) Removal of metals by Fe3O4, loaded activated carbon prepared from plum stone (Prunus nigra): kinetics and modelling study. Powder Technol 317:23–30
Sun FY, Wang XM, Li XY (2013) An innovative membrane bioreactor (MBR) system for simultaneous nitrogen and phosphorus removal. Process Biochem 48(11):1749–1756
Tan ZZ, Zhang XY, Wang CS (2019) Adsorption characteristics of amoxicillin in water by coconut shell carbon. Jiangsu Agric Sci 47(04):260–264
Wang J (2017) Study on the removal of nitrogen and phosphorus from water by zirconium metal/chitosan/bentonite complex. Dissertation, Northwest Agricultural and Forestry University of science and technology
Wang L, Chen ZZ (2018) Wen H (2018) Microwave assisted modification of activated carbons by organic acid ammoniums activation for enhanced adsorption of acid red 18. Powder Technol 323:230–237
Wang ZF, Nie E, Li J (2012) Equilibrium and kinetics of adsorption of phosphate onto iron-doped activated carbon. Environ Sci Pollut Res 19(7):2908–2917
Wasif F, Hong H-J, Eun JK (2012) Removal of bromate (BrO-3) from water using cationic surfactant-modified powdered activated carbon (SM-PAC). Sep Sci Technol 47:13,1906–13,1912
Xiong W, Tong J, Yang Z (2017) Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: performance and mechanism. J Colloid Interface Sci 493(Complete):17–23
Xu JH, Gao NY, Deng Y (2011) Perchlorate removal by granular activated carbon coated with cetyltrimethyl ammonium bromide. J Colloid Interface Sci 357(2):474–479
Yan LG, Wang J, Yu HQ (2007) Adsorption of benzoic acid by CTAB exchanged montmorillonite. Appl Clay Sci 37(3-4):0-230
Yang K, Fox J (2018) Adsorption of humic acid by acid-modified granular activated carbon and powder activated carbon. J Environ Eng
Zhan YH, Zhang HH, Lin JW (2017) Role of zeolite’s exchangeable cations in phosphate adsorption onto zirconium-modified zeolite. J Mol Liq S0167-7322(17):31714–31712
Zhang GF, Jiang XH, Cui YB (2005) Research and application of vetiver. Sci Grass Ind 1:73–78
Zhang GG, Xiao ZC, Zhang WM (2015) Study and utilization of vetiver. China Wild Plant Resour 2:70–74
Zhi MM, Wang PF, Hou ZY (2019) Effect of NH_4~+ on phosphorus removal by magnesium modified biochar. Environ Sci 40(02):669–676
Zhong L, Zhan HY, Hill DO (2000) Removal of ammonia nitrogen from wastewater by chemical precipitation and its reaction. Chongqing Environ Sci 22(6):54–56
Zhu JL, Wang XZ, Shen J (2017) Adsorption of nitrate and phosphate in water by activated carbon modified by tedecyl trimethylammonium bromide. Chem Prog 7
Funding
This work was supported by the Science Research Fund Projects of Department of Education of Yunnan Province (Grant No. 2020J0329) and the Science Research Fund Projects of Department of Education of Yunnan Province (Grant No. 2020J0327)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 237 kb)
Rights and permissions
About this article
Cite this article
Li, J., Fang, X., Yang, M. et al. The adsorption properties of functionalization vetiver grass-based activated carbon: the simultaneous adsorption of phosphate and nitrate. Environ Sci Pollut Res 28, 40544–40554 (2021). https://doi.org/10.1007/s11356-020-09271-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09271-5