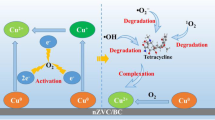
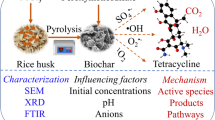
Abstract
The advanced oxidation processes (AOPs), especially sulphate radical (SO4•−)–based AOPs (SR-AOPs), have been considered more effective, selective, and prominent technologies for the removal of highly toxic emerging contaminants (ECs) due to wide operational pH range and relatively higher oxidation potential (2.5–3.1 V). Recently, biochar (BC)-based composite materials have been introduced in AOPs due to the dual benefits of adsorption and catalytic degradation, but the scientific review of BC-based catalysts for the generation of reactive oxygen species (ROSs) through radical- and non-radical-oriented routes for EC removal was rarely reported. The chemical treatments, such as acid/base treatment, chemical oxidation, surfactant incorporation, and coating and impregnation of minerals, were applied to make BC suitable as supporting materials (SMs) for the loading of Fenton catalysts to boost up peroxymonosulphate/persulphate/H2O2 activation to get ROSs including •OH, SO4•−, 1O2, and O2•− for targeted pollutant degradation. In this review, all the possible merits of BC-based catalysts including supportive, adsorptive, and catalytic role are summarised along with the possible route for the development prospects of BC properties. The limitations of SR-AOPs especially on production of non-desired oxyanions, as well as disinfection intermediates and their potential solutions, have been identified. Lastly, the knowledge gap and future-oriented research needs are highlighted.








Similar content being viewed by others
Abbreviations
- AC:
-
Activated carbon
- AOPs:
-
Advanced oxidation processes
- BC:
-
Biochar
- CNTs:
-
Carbon nanotubes
- CPC:
-
Cetylpyridinium chloride
- DOM:
-
Dissolved organic matter
- ECs:
-
Emerging contaminants
- EDCs:
-
Endocrine-disrupting chemicals
- FGs:
-
Functional groups
- HTT:
-
Heat treatment temperature
- O2•- :
-
Superoxide radicals
- SMZ:
-
Sulfamethoxazole
- RhB:
-
Rhodamine B
- SD:
-
Sulfadiazine
- PFRs:
-
Persistent free radicals
- MOFs:
-
Metal organic frameworks
- OFGs:
-
Oxygen functional groups
- PCP:
-
Pentachlorophenol
- PMS:
-
Peroxymonosulphate
- PDS:
-
Peroxydisulphate
- PS:
-
Persulphate
- HDBPs:
-
Harmful disinfection by-products
- ROSs:
-
Reactive oxygen species
- SMs:
-
Supporting materials
- SR-AOPs:
-
Sulphate radical–based AOPs
- TC:
-
Tetracycline
- IBF:
-
Ibuprofen
- NBCs:
-
N-doped biochars
References
Anipsitakis GP, Stathatos E, Dionysiou DD (2005) Heterogeneous activation of oxone using Co3O4. J Phys Chem B 109:13052–13055. https://doi.org/10.1021/jp052166y
Anipsitakis GP, Dionysiou DD, Gonzalez MA (2006) Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ Sci Technol 40:1000–1007
Asghar A, Raman AAA, Daud WMAW (2015) Advanced oxidation processes for in situ production of hydrogen peroxide/hydroxyl radical. https://doi.org/10.1016/j.jclepro.2014.09.010
Azargohar R, Dalai AK (2008) Steam and KOH activation of biochar: experimental and modeling studies. Microporous Mesoporous Mater 110:413–421. https://doi.org/10.1016/j.micromeso.2007.06.047
Bautista P, Mohedano A, Casas J, Zazo J, Rodriguez J (2010) Oxidation of cosmetic wastewaters with H2O2 using a Fe/γ-Al2O3 catalyst. Water Sci Technol 61:1631–1636
Bennedsen LR, Muff J, Søgaard EG (2012) Influence of chloride and carbonates on the reactivity of activated persulfate. Chemosphere 86:1092–1097
Boix C et al (2016) Behaviour of emerging contaminants in sewage sludge after anaerobic digestion. Chemosphere 163:296–304. https://doi.org/10.1016/j.chemosphere.2016.07.098
Cai X et al (2018) Titanium dioxide-coated biochar composites as adsorptive and photocatalytic degradation materials for the removal of aqueous organic pollutants. J Chem Technol Biotechnol 93:783–791
Carrier A NC, Oakley D, Chen Y, Oakes K, MacQuarrie S, Xu Zhang (2018) Selective generation of singlet oxygen in chloride accelerated copper fenton chemistry. https://chemrxiv.org/articles/Selective_Generation_of_Singlet_Oxygen_in_Chloride_Accelerated_Copper_Fenton_Chemistry/7364225/1. Accessed 4 Dec 2019
Cazetta AL, Martins AC, Pezoti O, Bedin KC, Beltrame KK, Asefa T, Almeida VC (2016) Synthesis and application of N–S-doped mesoporous carbon obtained from nanocasting method using bone char as heteroatom precursor and template. Chem Eng J 300:54–63
Chan KH, Chu W (2009) Degradation of atrazine by cobalt-mediated activation of peroxymonosulfate: different cobalt counteranions in homogenous process and cobalt oxide catalysts in photolytic heterogeneous process. Water Res 43:2513–2521
Chen C, Li X, Tong Z, Li Y, Li M (2014) Modification process optimization, characterization and adsorption property of granular fir-based activated carbon. Appl Surf Sci 315:203–211
Chen N, Huang Y, Hou X, Ai Z, Zhang L (2017) Photochemistry of hydrochar: reactive oxygen species generation and sulfadimidine degradation. Environ Sci Technol 51:11278–11287
Chen L, Yang S, Zuo X, Huang Y, Cai T, Ding D (2018) Biochar modification significantly promotes the activity of Co3O4 towards heterogeneous activation of peroxymonosulfate. Chem Eng J 354:856–865
Cheng X, Guo H, Zhang Y, Wu X, Liu Y (2017) Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res 113:80–88
Chi GT, Churchley J, Huddersman KD (2013) Pilot-scale removal of trace steroid hormones and pharmaceuticals and personal care products from municipal wastewater using a heterogeneous Fenton’s catalytic process. Int J Chem Eng 2013:1–10. https://doi.org/10.1155/2013/760915
Criquet J, Leitner NK (2009) Degradation of acetic acid with sulfate radical generated by persulfate ions photolysis. Chemosphere 77:194–200
Dantas T, Mendonca V, Jose H, Rodrigues A, Moreira R (2006) Treatment of textile wastewater by heterogeneous Fenton process using a new composite Fe2O3/carbon. Chem Eng J 118:77–82
Deng D, Peng L, Guan M, Kang Y (2014) Impact of activation methods on persulfate oxidation of methyl tert-butyl ether. J Hazard Mater 264:521–528
Deng J et al (2018) Nanoscale zero-valent iron/biochar composite as an activator for Fenton-like removal of sulfamethazine. Sep Purif Technol 202:130–137. https://doi.org/10.1016/j.seppur.2018.03.048
Devi P, Saroha AK (2014) Synthesis of the magnetic biochar composites for use as an adsorbent for the removal of pentachlorophenol from the effluent. Bioresour Technol 169:525–531
Devi P, Saroha AK (2015) Simultaneous adsorption and dechlorination of pentachlorophenol from effluent by Ni–ZVI magnetic biochar composites synthesized from paper mill sludge. Chem Eng J 271:195–203
Ding ZH, Hu X, Zimmerman AR, Gao B (2014) Sorption and cosorption of lead (II) and methylene blue on chemically modified biomass. Bioresour Technol 167:569–573. https://doi.org/10.1016/j.biortech.2014.06.043
Dong X, Ma LQ, Gress J, Harris W, Li Y (2014) Enhanced Cr (VI) reduction and As (III) oxidation in ice phase: important role of dissolved organic matter from biochar. J Hazard Mater 267:62–70
Duan XG, Sun HQ, Kang J, Wang YX, Indrawirawan S, Wang SB (2015) Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons. ACS Catal 5:4629–4636. https://doi.org/10.1021/acscatal.5b00774
Duan X, Ao Z, Zhou L, Sun H, Wang G, Wang S (2016) Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation. Appl Catal B Environ 188:98–105
Erdinc N, Gokturk S, Tuncay M (2010) A study on the adsorption characteristics of an amphiphilic phenothiazine drug on activated charcoal in the presence of surfactants. Colloid Surf B 75:194–203. https://doi.org/10.1016/j.colsurfb.2009.08.031
Faheem YH, Liu J, Shen J, Sun X, Li J, Wang L (2016) Preparation of MnOx-loaded biochar for Pb2+ removal: adsorption performance and possible mechanism. J Taiwan Inst Chem Eng 66:313–320
Faheem, Bao JG, Zheng H, Tufail H, Irshad S, Du JK (2018) Adsorption-assisted decontamination of Hg(II) from aqueous solution by multi-functionalized corncob-derived biochar. RSC Adv 8:38425–38435. https://doi.org/10.1039/c8ra06622a
Faheem, Du JK, Bao JG, Hassan MA, Irshad S, Talib MA (2019) Multi-functional biochar novel surface chemistry for efficient capture of anionic Congo red dye: behavior and mechanism. Arab J Sci Eng 44:10127–10139. https://doi.org/10.1007/s13369-019-04194-x
Fan Y, Wang B, Yuan S, Wu X, Chen J, Wang L (2010) Adsorptive removal of chloramphenicol from wastewater by NaOH modified bamboo charcoal. Bioresour Technol 101:7661–7664
Fang G, Gao J, Liu C, Dionysiou DD, Wang Y, Zhou D (2014) Key role of persistent free radicals in hydrogen peroxide activation by biochar: implications to organic contaminant degradation. Environ Sci Technol 48:1902–1910
Fang G, Liu C, Gao J, Dionysiou DD, Zhou D (2015a) Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ Sci Technol 49:5645–5653
Fang G, Zhu C, Dionysiou DD, Gao J, Zhou D (2015b) Mechanism of hydroxyl radical generation from biochar suspensions: implications to diethyl phthalate degradation. Bioresour Technol 176:210–217
Fang GD, Liu C, Wang YJ, Dionysiou DD, Zhou DM (2017) Photogeneration of reactive oxygen species from biochar suspension for diethyl phthalate degradation. Appl Catal B Environ 214:34–45. https://doi.org/10.1016/j.apcatb.2017.05.036
Feng Z, Zhu L (2017) Sorption of phenanthrene to biochar modified by base. Front Environ Sci Eng 12:1
Fu H et al (2016) Photochemistry of dissolved black carbon released from biochar: reactive oxygen species generation and phototransformation. Environ Sci Technol 50:1218–1226
Fu D, Chen Z, Xia D, Shen L, Wang Y, Li Q (2017) A novel solid digestate-derived biochar-Cu NP composite activating H2O2 system for simultaneous adsorption and degradation of tetracycline. Environ Pollut 221:301–310. https://doi.org/10.1016/j.envpol.2016.11.078
Fujita I, Tomooka J, Sugimura T (1991) Sorption of anionic surfactants with wood charcoal. Bull Chem Soc Jpn 64:738–740
Gao H, Chen J, Zhang Y, Zhou X (2016) Sulfate radicals induced degradation of triclosan in thermally activated persulfate system. Chem Eng J 306:522–530
Gao Y, Pramanik A, Begum S, Sweet C, Jones S, Alamgir A, Ray PC (2017) Multifunctional biochar for highly efficient capture, identification, and removal of toxic metals and superbugs from water samples. ACS Omega 2:7730–7738. https://doi.org/10.1021/acsomega.7b01386
Georgi A, Kopinke F-D (2005) Interaction of adsorption and catalytic reactions in water decontamination processes: part I. oxidation of organic contaminants with hydrogen peroxide catalyzed by activated carbon. Appl Catal B Environ 58:9–18
Gerken JB, McAlpin JG, Chen JY, Rigsby ML, Casey WH, Britt RD, Stahl SS (2011) Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0–14: the thermodynamic basis for catalyst structure, stability, and activity. J Am Chem Soc 133:14431–14442
Ghaffar A, Younis MN (2014) Adsorption of organic chemicals on graphene coated biochars and its environmental implications. Green Process Synth 3:479–487
Grebel JE, Pignatello JJ, Mitch WA (2010) Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters. Environ Sci Technol 44:6822
Greluk M, Hubicki Z (2010) Kinetics, isotherm and thermodynamic studies of Reactive Black 5 removal by acid acrylic resins. Chem Eng J 162:919–926
Grover D, Zhou J, Frickers P, Readman J (2011) Improved removal of estrogenic and pharmaceutical compounds in sewage effluent by full scale granular activated carbon: impact on receiving river water. J Hazard Mater 185:1005–1011
Guan Y-H, Ma J, Ren Y-M, Liu Y-L, Xiao J-Y, Lin L-q, Zhang C (2013) Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res 47:5431–5438
Guan XJ, Zhou J, Ma N, Chen XY, Gao JQ, Zhang RQ (2015) Studies on modified conditions of biochar and the mechanism for fluoride removal. Desalin Water Treat 55:440–447. https://doi.org/10.1080/19443994.2014.916230
Hamid SBA, Chowdhury ZZ, Zain SM (2014) Base catalytic approach: a promising technique for the activation of biochar for equilibrium sorption studies of copper, Cu (II) ions in single solute system. Materials 7:2815–2832
Han D, Liu X, Zhang G, Sun K, Jiao Y (2013) Effects of cationic surfactant on pentachlorophenol sorption by sediment, active carbon and biochar. Fresenius Environ Bull 22:1280–1286
Han L, Xue S, Zhao S, Yan J, Qian L, Chen M (2015a) Biochar supported nanoscale iron particles for the efficient removal of methyl orange dye in aqueous solutions. PLoS One 10:e0132067
Han XY et al (2015b) Removal of methylene blue from aqueous solution using porous biochar obtained by KOH activation of peanut shell biochar. Bioresources 10:2836–2849. https://doi.org/10.15376/biores.10.2.2836-2849
Hazime R, Nguyen Q, Ferronato C, Salvador A, Jaber F, Chovelon J-M (2014) Comparative study of imazalil degradation in three systems: UV/TiO2, UV/K2S2O8 and UV/TiO2/K2S2O8. Appl Catal B Environ 144:286–291
Hu WR, Xie Y, Lu S, Li PY, Xie TH, Zhang YK, Wang YB (2019) One-step synthesis of nitrogen-doped sludge carbon as a bifunctional material for the adsorption and catalytic oxidation of organic pollutants. Sci Total Environ 680:51. https://doi.org/10.1016/j.scitotenv.2019.05.098
Huang B-C, Jiang J, Huang G-X, Yu H-Q (2018) Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate. J Mater Chem A 6:8978–8985. https://doi.org/10.1039/C8TA02282H
Huff MD, Lee JW (2016) Biochar-surface oxygenation with hydrogen peroxide. J Environ Manag 165:17–21. https://doi.org/10.1016/j.jenvman.2015.08.046
Hussain I et al (2017) Insights into the mechanism of persulfate activation with nZVI/BC nanocomposite for the degradation of nonylphenol. Chem Eng J 311:163–172
Hutchings GS (2015) Advanced nanostructured materials for energy storage and conversion. University of Delaware, PhD diss
Inyang M, Dickenson E (2015) The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: a review. Chemosphere 134:232
Inyang M, Gao B, Zimmerman A, Zhang M, Chen H (2014) Synthesis, characterization, and dye sorption ability of carbon nanotube–biochar nanocomposites. Chem Eng J 236:39–46
Inyang M, Gao B, Zimmerman A, Zhou Y, Cao X (2015) Sorption and cosorption of lead and sulfapyridine on carbon nanotube-modified biochars. Environ Sci Pollut Res 22:1868–1876
Jafari AJ, Kakavandi B, Jaafarzadeh N, Kalantary RR, Ahmadi M, Babaei AA (2017) Fenton-like catalytic oxidation of tetracycline by AC@ Fe3O4 as a heterogeneous persulfate activator: adsorption and degradation studies. J Ind Eng Chem 45:323–333
Jeon P, Lee M-E, Baek K (2017a) Adsorption and photocatalytic activity of biochar with graphitic carbon nitride (g-C3N4). J Taiwan Inst Chem Eng 77:244–249
Jeon P, Lee ME, Baek K (2017b) Adsorption and photocatalytic activity of biochar with graphitic carbon nitride (g-C 3 N 4 ). J Taiwan Inst Chem Eng 77:244–249
Jiang M, Lu J, Ji Y, Kong D (2017) Bicarbonate-activated persulfate oxidation of acetaminophen. Water Res 116:324–331
Jing X-R, Wang Y-Y, Liu W-J, Wang Y-K, Jiang H (2014) Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem Eng J 248:168–174
Jung C et al (2013) Adsorption of selected endocrine disrupting compounds and pharmaceuticals on activated biochars. J Hazard Mater 263:702–710. https://doi.org/10.1016/j.jhazmat.2013.10.033
Jung C, Boateng LK, Flora JRV, Oh J, Braswell MC, Son A, Yoon Y (2015) Competitive adsorption of selected non-steroidal anti-inflammatory drugs on activated biochars: experimental and molecular modeling study. Chem Eng J 264:1–9
Kearns JP, Shimabuku KK, Mahoney RB, Knappe DRU, Summers RS (2015) Meeting multiple water quality objectives through treatment using locally generated char: improving organoleptic properties and removing synthetic organic contaminants and disinfection by-products. J Water Sanit Hyg De 5:359–372. https://doi.org/10.2166/washdev.2015.172
Keen OS, Mckay G, Mezyk SP, Linden KG, Rosario-Ortiz FL (2014) Identifying the factors that influence the reactivity of effluent organic matter with hydroxyl radicals. Water Res 50:408–419
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253
Kemmou L, Frontistis Z, Vakros J, Manariotis ID, Mantzavinos D (2018) Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: factors affecting the activation and degradation processes. Catal Today 313:128–133
Khataee A, Kayan B, Gholami P, Kalderis D, Akay S (2017) Sonocatalytic degradation of an anthraquinone dye using TiO2-biochar nanocomposite. Ultrason Sonochem 39:120–128
Khataee A, Gholami P, Kalderis D, Pachatouridou E, Konsolakis M (2018) Preparation of novel CeO2-biochar nanocomposite for sonocatalytic degradation of a textile dye. Ultrason Sonochem 41:503–513
Kim D, Park S, Park KY (2017) Upgrading the fuel properties of sludge and low rank coal mixed fuel through hydrothermal carbonization. Energy 141:598–602
Kim J, Park B, Son Y, Khim J (2018) Peat moss-derived biochar for sonocatalytic applications. Ultrason Sonochem 42:26–30. https://doi.org/10.1016/j.ultsonch.2017.11.005
Kirisits MJ, Snoeyink VL, Kruithof JC (2000) The reduction of bromate by granular activated carbon. Water Res 34:4250–4260
Klüpfel L, Keiluweit M, Kleber M, Sander M (2014) Redox properties of plant biomass-derived black carbon (biochar). Environ Sci Technol 48:5601–5611
Kong L et al (2014) Sorption performance and mechanism of a sludge-derived char as porous carbon-based hybrid adsorbent for benzene derivatives in aqueous solution. J Hazard Mater 274:205–211
Kunde GB, Yadav GD (2015) Synthesis, characterization and application of iron-aluminate nodules in advanced Fenton’s oxidation process. J Environ Chem Eng 3:2010–2021
Lan H, Wang A, Liu R, Liu H, Qu J (2015) Heterogeneous photo-Fenton degradation of acid red B over Fe2O3 supported on activated carbon fiber. J Hazard Mater 285:167–172
Lee Y-C, Lo S-L, Chiueh P-T, Chang D-G (2009) Efficient decomposition of perfluorocarboxylic acids in aqueous solution using microwave-induced persulfate. Water Res 43:2811–2816
Lee Y-C, Lo S-L, Kuo J, Huang C-P (2013) Promoted degradation of perfluorooctanic acid by persulfate when adding activated carbon. J Hazard Mater 261:463–469
Li J, Li Y, Wu Y, Zheng M (2014a) A comparison of biochars from lignin, cellulose and wood as the sorbent to an aromatic pollutant. J Hazard Mater 280:450–457
Li J, Lv G, Bai W, Liu Q, Zhang Y, Song J (2014b) Modification and use of biochar from wheat straw (Triticum aestivum L.) for nitrate and phosphate removal from water. Desalin Water Treat 57:1–13. https://doi.org/10.1080/19443994.2014.994104
Li Y, Shao J, Wang X, Deng Y, Yang H, Chen H (2014c) Characterization of modified biochars derived from bamboo pyrolysis and their utilization for target component (furfural) adsorption. Energy Fuel 28:5119–5127. https://doi.org/10.1021/ef500725c
Li Z, Chen Z, Xiang Y, Ling L, Fang J, Shang C, Dionysiou DD (2015) Bromate formation in bromide-containing water through the cobalt-mediated activation of peroxymonosulfate. Water Res 83:132–140
Li A, Lin RJ, Lin C, He BY, Zheng TT, Lu LB, Cao Y (2016) An environment-friendly and multi-functional absorbent from chitosan for organic pollutants and heavy metal ion. Carbohydr Polym 148:272–280. https://doi.org/10.1016/j.carbpol.2016.04.070
Liang CJ, Bruell CJ, Marley MC, Sperry KL (2003) Thermally activated persulfate oxidation of trichloroethylene (TCE) and 1, 1, 1-trichloroethane (TCA) in aqueous systems and soil slurries. Soil Sediment Contam Int J 12:207–228
Liang C, Wang ZS, Mohanty N (2006) Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20 degrees C. Sci Total Environ 370:271–277
Liang C, Wang Z-S, Bruell CJ (2007) Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 66:106–113
Liu P, Liu W-J, Jiang H, Chen J-J, Li W-W, Yu H-Q (2012) Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour Technol 121:235–240
Liu K, Lu J, Ji Y (2015) Formation of brominated disinfection by-products and bromate in cobalt catalyzed peroxymonosulfate oxidation of phenol. Water Res 84:1–7
Liu XQ, Chen WJ, Jiang H (2017) Facile synthesis of Ag/Ag3PO4/AMB composite with improved photocatalytic performance. Chem Eng J 308:889–896. https://doi.org/10.1016/j.cej.2016.09.125
Liu Z et al (2018) Functionalizing bottom ash from biomass power plant for removing methylene blue from aqueous solution. Sci Total Environ 634:760–768. https://doi.org/10.1016/j.scitotenv.2018.04.010
Lomnicki S, Truong H, Vejerano E, Dellinger B (2008) Copper oxide-based model of persistent free radical formation on combustion-derived particulate matter. Environ Sci Technol 42:4982–4988. https://doi.org/10.1021/es071708h
Luo S et al (2017) Mechanistic insight into reactivity of sulfate radical with aromatic contaminants through single-electron transfer pathway. Chem Eng J 327:1056–1065. https://doi.org/10.1016/j.cej.2017.06.179
Luo M, Lin H, Li B, Dong Y, He Y, Wang L (2018) A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water. Bioresour Technol 259:312–318
Luo K, Yang Q, Pang Y, Wang D, Li X, Lei M, Huang Q (2019) Unveiling the mechanism of biochar-activated hydrogen peroxide on the degradation of ciprofloxacin. Chem Eng J 374:520–530. https://doi.org/10.1016/j.cej.2019.05.204
Mahmoud ME, Nabil GM, El-Mallah NM, Bassiouny HI, Kumar S, Abdel-Fattah TM (2016) Kinetics, isotherm, and thermodynamic studies of the adsorption of reactive red 195 A dye from water by modified Switchgrass Biochar adsorbent. J Ind Eng Chem 37:156–167
Manyà JJ, Azuara M, Manso JA (2018) Biochar production through slow pyrolysis of different biomass materials: seeking the best operating conditions. Biomass Bioenergy 117:115–123. https://doi.org/10.1016/j.biombioe.2018.07.019
Masel RI (1996) Principles of adsorption and reaction on solid surfaces, vol 3. John Wiley & Sons, Canada
Méndezdíaz J, Sánchezpolo M, Riverautrilla J, Canonica S, Gunten UV (2010) Advanced oxidation of the surfactant SDBS by means of hydroxyl and sulphate radicals. Chem Eng J 163:300–306
Meri NH, Alias AB, Talib N, Rashid ZA, Ghani WAWA (2018) Effect of chemical washing pre-treatment of empty fruit bunch (EFB) biochar on characterization of hydrogel biochar composite as bioadsorbent. Paper presented at the 3rd International Conference on Global Sustainability and Chemical Engineering (Icgsce)
Mertens R, von Sonntag C (1995) Photolysis (λ = 354 nm of tetrachloroethene in aqueous solutions). J Photochem Photobiol A Chem 85:1–9
Mi X, Li G, Zhu W, Liu L (2016) Enhanced adsorption of orange II using cationic surfactant modified biochar pyrolyzed from cornstalk. J Chemother 2016:1–7. https://doi.org/10.1155/2016/8457030
Mian MM, Liu G (2018) Recent progress in biochar-supported photocatalysts: synthesis, role of biochar, and applications. RSC Adv 8:14237–14248
Muhammad S, Shukla PR, Tadé MO, Wang S (2012) Heterogeneous activation of peroxymonosulphate by supported ruthenium catalysts for phenol degradation in water. J Hazard Mater 215:183–190
Nawi M, Jawad AH, Sabar S, Ngah WW (2011) Immobilized bilayer TiO2/chitosan system for the removal of phenol under irradiation by a 45 watt compact fluorescent lamp. Desalination 280:288–296
Neta P, Madhavan V, Zemel H, Fessenden RW (1977) Rate constants and mechanism of reaction of sulfate radical anion with aromatic compounds. J Am Chem Soc 99:163–164
Novak J, Ro K, Ok YS, Sigua G, Spokas K, Uchimiya S, Bolan N (2016) Biochars multifunctional role as a novel technology in the agricultural, environmental, and industrial sectors. Chemosphere 142:1–3
Ok YS, Chang SX, Gao B, Chung HJ (2015) SMART biochar technology-a shifting paradigm towards advanced materials and healthcare research. Environ Technol Innov 4:206–209. https://doi.org/10.1016/j.eti.2015.08.003
Okay O (2009) General properties of hydrogels. In: Hydrogel sensors and actuators. Springer Series on Chemical Sensors and Biosensors. Springer, Berlin, pp 1–14. https://doi.org/10.1007/978-3-540-75645-3_1
Oladipo AA, Ifebajo AO (2017) Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: two-stage adsorber analysis. J Environ Manag 209:9–16
Ouyang D et al (2017) Degradation of 1, 4-dioxane by biochar supported nano magnetite particles activating persulfate. Chemosphere 184:609–617
Paria S (2008) Surfactant-enhanced remediation of organic contaminated soil and water. Adv Colloid Interf Sci 138:24–58
Park JH, Wang JJ, Xiao R, Tafti N, DeLaune RD, Seo DC (2018) Degradation of orange G by Fenton-like reaction with Fe-impregnated biochar catalyst. Bioresour Technol 249:368–376. https://doi.org/10.1016/j.biortech.2017.10.030
Peng X et al (2017) New insights into the activity of a biochar supported nanoscale zerovalent iron composite and nanoscale zero valent iron under anaerobic or aerobic conditions. RSC Adv 7:8755–8761
Pi L, Jiang R, Zhou W, Zhu H, Xiao W, Wang D, Mao X (2015) g-C 3 N 4 modified biochar as an adsorptive and photocatalytic material for decontamination of aqueous organic pollutants. Appl Surf Sci 358:231–239
Pi ZJ et al (2019) Persulfate activation by oxidation biochar supported magnetite particles for tetracycline removal: performance and degradation pathway. J Clean Prod 235:1103–1115. https://doi.org/10.1016/j.jclepro.2019.07.037
Prieto-Rodríguez L, Oller I, Klamerth N, Agüera A, Rodríguez E, Malato S (2013) Application of solar AOPs and ozonation for elimination of micropollutants in municipal wastewater treatment plant effluents. Water Res 47:1521–1528
Qin J, Chen Q, Sun M, Sun P, Shen G (2017) Pyrolysis temperature-induced changes in the catalytic characteristics of rice husk-derived biochar during 1, 3-dichloropropene degradation. Chem Eng J 330:804–812
Rajapaksha AU et al (2016) Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification. Chemosphere 148:276–291. https://doi.org/10.1016/j.chemosphere.2016.01.043
Rastogi A, Al-Abed SR, Dionysiou DD (2009a) Effect of inorganic, synthetic and naturally occurring chelating agents on Fe(II) mediated advanced oxidation of chlorophenols. Water Res 43:684–694
Rastogi A, Al-Abed SR, Dionysiou DD (2009b) Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl Catal B Environ 85:171–179
Regmi P, Moscoso JLG, Kumar S, Cao X, Mao J, Schafran G (2012) Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J Environ Manag 109:61–69
Saleh MM (2006) On the removal of cationic surfactants from dilute streams by granular charcoal. Water Res 40:1052–1060
Santhanalakshmi J, Balaji S (1996) Adsorption studies of nonionic surfactants on charcoal and alumina in aromatic solvents. J Colloid Interface Sci 179:517–521
Setianingsih T, Masruri M, Ismuyanto B (2018) Synthesis of patchouli biochar Cr2O3 composite using double acid oxidators for paracetamol adsorption. J Pure App Chem Res 7:60–69
Shen W, Li Z, Liu Y (2008) Surface chemical functional groups modification of porous carbon. Recent Patents Chem Eng 1:27–40
Shen B, Li G, Wang F, Wang Y, He C, Zhang M, Singh S (2015) Elemental mercury removal by the modified bio-char from medicinal residues. Chem Eng J 272:28–37
Snyder SA et al (2007) Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 202:156–181
Song R, Bai B, Puma GL, Wang H, Suo Y (2015) Biosorption of azo dyes by raspberry-like Fe3O4@ yeast magnetic microspheres and their efficient regeneration using heterogeneous Fenton-like catalytic processes over an up-flow packed reactor. React Kinet Mech Catal 115:547–562
Sun L, Chen D, Wan S, Yu Z (2015) Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour Technol 198:300–308
Sun H, Peng X, Zhang S, Liu S, Xiong Y, Tian S, Fang J (2017) Activation of peroxymonosulfate by nitrogen-functionalized sludge carbon for efficient degradation of organic pollutants in water. Bioresour Technol 241:244–251
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y, Yang Z (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85
Tan XF et al (2016) Biochar-based nano-composites for the decontamination of wastewater: a review. Bioresour Technol 212:318–333. https://doi.org/10.1016/j.biortech.2016.04.093
Tang L et al (2017) Treatment of arsenic in acid wastewater and river sediment by Fe@ Fe2O3 nanobunches: the effect of environmental conditions and reaction mechanism. Water Res 117:175–186
Tang L et al (2018) Sustainable efficient adsorbent: alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem Eng J 336:160–169. https://doi.org/10.1016/j.cej.2017.11.048
Toor R, Mohseni M (2007) UV-H2O2 based AOP and its integration with biological activated carbon treatment for DBP reduction in drinking water. Chemosphere 66:2087–2095
Tully F, Ravishankara A, Thompson R, Nicovich J, Shah R, Kreutter N, Wine P (1981) Kinetics of the reactions of hydroxyl radical with benzene and toluene. J Phys Chem 85:2262–2269
Uchimiya M, Lima IM, Klasson KT, Wartelle LH (2010) Contaminant immobilization and nutrient release by biochar soil amendment: roles of natural organic matter. Chemosphere 80:935–940
Usman M, Faure P, Ruby C, Hanna K (2012) Application of magnetite-activated persulfate oxidation for the degradation of PAHs in contaminated soils. Chemosphere 87:234–240
Vejerano E, Lomnicki S, Dellinger B (2010) Formation and stabilization of combustion-generated environmentally persistent free radicals on an Fe (III)2O3/silica surface. Environ Sci Technol 45:589–594. https://doi.org/10.1021/es102841s
Von Sonntag C, Von Gunten U (2012) Chemistry of ozone in water and wastewater treatment. IWA publishing, London
Wang GS, Hsieh ST, Hong CS (2000) Destruction of humic acid in water by UV light—catalyzed oxidation with hydrogen peroxide. Water Res 34:3882–3887
Wang Z, Yuan R, Guo Y, Xu L, Liu J (2011) Effects of chloride ions on bleaching of azo dyes by Co2+/oxone reagent: kinetic analysis. J Hazard Mater 190:1083–1087
Wang Y, Le Roux J, Zhang T, Croué J-P (2014) Formation of brominated disinfection byproducts from natural organic matter isolates and model compounds in a sulfate radical-based oxidation process. Environ Sci Technol 48:14534–14542
Wang B, Lehmann J, Hanley K, Hestrin R, Enders A (2015) Adsorption and desorption of ammonium by maple wood biochar as a function of oxidation and pH. Chemosphere 138:120–126
Wang B, Lehmann J, Hanley K, Hestrin R, Enders A (2016a) Ammonium retention by oxidized biochars produced at different pyrolysis temperatures and residence times. RSC Adv 6:41907–41913
Wang Q et al (2016b) Degradation kinetics and mechanism of 2,4-di-tert-butylphenol with UV/persulfate. Chem Eng J 304:201–208. https://doi.org/10.1016/j.cej.2016.06.092
Wang Y, Ao Z, Sun H, Duan X, Wang S (2016c) Activation of peroxymonosulfate by carbonaceous oxygen groups: experimental and density functional theory calculations. Appl Catal B Environ 198:295–302
Wang G, Chen S, Quan X, Yu H, Zhang Y (2017a) Enhanced activation of peroxymonosulfate by nitrogen doped porous carbon for effective removal of organic pollutants. Carbon 115:730–739
Wang J et al (2017b) Treatment of refractory contaminants by sludge-derived biochar/persulfate system via both adsorption and advanced oxidation process. Chemosphere 185:754–763. https://doi.org/10.1016/j.chemosphere.2017.07.084
Wang HZ et al (2019) Edge-nitrogenated biochar for efficient peroxydisulfate activation: an electron transfer mechanism. Water Res 160:405–414. https://doi.org/10.1016/j.watres.2019.05.059
Wu Y, Guo J, Han Y, Zhu J, Zhou L, Lan Y (2018) Insights into the mechanism of persulfate activated by rice straw biochar for the degradation of aniline. Chemosphere 200:373–379
Xia D et al (2016) ZnCl 2 -activated biochar from biogas residue facilitates aqueous As(III) removal. Appl Surf Sci 377:361–369
Xiao R, Ye T, Wei Z, Luo S, Yang Z, Spinney R (2015) Quantitative structure–activity relationship (QSAR) for the oxidation of trace organic contaminants by sulfate radical. Environ Sci Technol 49:13394–13402
Xie M, Chen W, Xu Z, Zheng S, Zhu D (2014) Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ Pollut 186:187–194. https://doi.org/10.1016/j.envpol.2013.11.022
Xu X, Zhao Y, Sima J, Zhao L, Mašek O, Cao X (2017) Indispensable role of biochar-inherent mineral constituents in its environmental applications: a review. Bioresour Technol 241:887–899
Xue Y, Gao B, Yao Y, Inyang M, Zhang M, Zimmerman AR, Ro KS (2012) Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: batch and column tests. Chem Eng J 200:673–680
Yakout SM (2015) Monitoring the changes of chemical properties of rice straw-derived biochars modified by different oxidizing agents and their adsorptive performance for organics. Bioremediat J 19:171–182
Yan L, Kong L, Qu Z, Li L, Shen G (2014) Magnetic biochar decorated with ZnS nanocrytals for Pb (II) removal. ACS Sustain Chem Eng 3:125–132
Yan J, Han L, Gao W, Xue S, Chen M (2015) Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour Technol 175:269–274
Yan J, Qian L, Gao W, Chen Y, Ouyang D, Chen M (2017) Enhanced Fenton-like degradation of trichloroethylene by hydrogen peroxide activated with nanoscale zero valent iron loaded on biochar. Sci Rep 7:43051
Yang G-X, Jiang H (2014) Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res 48:396–405
Yang S, Yang X, Shao X, Niu R, Wang L (2011) Activated carbon catalyzed persulfate oxidation of azo dye acid orange 7 at ambient temperature. J Hazard Mater 186:659–666
Yang G, Chen H, Qin H, Feng Y (2014) Amination of activated carbon for enhancing phenol adsorption: effect of nitrogen-containing functional groups. Appl Surf Sci 293:299–305
Yang J, Pan B, Li H, Liao S, Zhang D, Wu M, Xing B (2015) Degradation of p-nitrophenol on biochars: role of persistent free radicals. Environ Sci Technol 50:694–700
Yao Y, Gao B, Chen J, Yang L (2013) Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer. Environ Sci Technol 47:8700–8708
Yao Y et al (2014) Characterization and environmental applications of clay–biochar composites. Chem Eng J 242:136–143
Yen CH, Chen KF, Kao CM, Liang SH, Chen TY (2011) Application of persulfate to remediate petroleum hydrocarbon-contaminated soil: feasibility and comparison with common oxidants. J Hazard Mater 186:2097–2102. https://doi.org/10.1016/j.jhazmat.2010.12.129
Yu W, Lian F, Cui G, Liu Z (2018) N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 193:8–16
Yu J et al (2019a) Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: Internal electron transfer mechanism. Chem Eng J 364:146–159
Yu JF et al (2019b) Hierarchical porous biochar from shrimp shell for persulfate activation: a two-electron transfer path and key impact factors. Appl Catal B Environ 260. https://doi.org/10.1016/j.apcatb.2019.118160
Zazo J, Casas J, Mohedano A, Rodríguez J (2006) Catalytic wet peroxide oxidation of phenol with a Fe/active carbon catalyst. Appl Catal B Environ 65:261–268
Zeng T, Zhang X, Wang S, Niu H, Cai Y (2014) Spatial confinement of Co3O4 catalyst in hollow metal-organic framework as nanoreactor for improved degradation of organic pollutant. Environ Sci Technol 49:2350
Zhang M, Gao B, Yao Y, Xue Y, Inyang M (2012) Synthesis, characterization, and environmental implications of graphene-coated biochar. Sci Total Environ 435-436:567–572
Zhang XN, Mao GY, Jiao YB, Shang Y, Han RP (2014) Adsorption of anionic dye on magnesium hydroxide-coated pyrolytic bio-char and reuse by microwave irradiation. Int J Environ Sci Technol (Tehran) 11:1439–1448
Zhang B-T, Zhang Y, Teng Y, Fan M (2015) Sulfate radical and its application in decontamination technologies. Crit Rev Environ Sci Technol 45:1756–1800
Zhang Y, Liu C, Xu B, Qi F, Chu W (2016) Degradation of benzotriazole by a novel Fenton-like reaction with mesoporous Cu/MnO2: combination of adsorption and catalysis oxidation. Appl Catal B Environ 199:447–457
Zhang H, Wang Z, Li R, Guo J, Li Y, Zhu J, Xie X (2017) TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere 185:351–360
Zhang K, Sun P, Faye MCAS, Zhang Y (2018a) Characterization of biochar derived from rice husks and its potential in chlorobenzene degradation. Carbon 130:730–740
Zhang Y, Cao B, Zhao L, Sun L, Gao Y, Li J, Yang F (2018b) Biochar-supported reduced graphene oxide composite for adsorption and coadsorption of atrazine and lead ions. Appl Surf Sci 427:147–155
Zhang C, Zhang N, Xiao Z, Li Z, Zhang D (2019a) Characterization of biochars derived from different materials and their effects on microbial dechlorination of pentachlorophenol in a consortium. RSC Adv 9:917–923. https://doi.org/10.1039/C8RA09410A
Zhang P et al (2019b) Catalytic degradation of estrogen by persulfate activated with iron-doped graphitic biochar: process variables effects and matrix effects. Chem Eng J:378. https://doi.org/10.1016/j.cej.2019.122141
Zhou J, Zhang Z, Banks E, Grover D, Jiang J (2009) Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. J Hazard Mater 166:655–661
Zhou Y, Gao B, Zimmerman AR, Fang J, Sun Y, Cao X (2013) Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem Eng J 231:512–518
Zhou X et al (2019) Persulfate activation by swine bone char-derived hierarchical porous carbon: multiple mechanism system for organic pollutant degradation in aqueous media. Chem Eng J: https://doi.org/10.1016/j.cej.2019.123091
Zhu B, Fan T, Zhang D (2008) Adsorption of copper ions from aqueous solution by citric acid modified soybean straw. J Hazard Mater 153:300
Zhu S, Huang X, Ma F, Wang L, Duan X, Wang S (2018) Catalytic removal of aqueous contaminants on n-doped graphitic biochars: inherent roles of adsorption and nonradical mechanisms. Environ Sci Technol 52:8649–8658
Zou Y, Li W, Yang L, Xiao F, An G, Wang Y, Wang D (2019) Activation of peroxymonosulfate by sp2-hybridized microalgae-derived carbon for ciprofloxacin degradation: importance of pyrolysis temperature. Chem Eng J 370:1286–1297
Funding
This research is financed by the Hubei Natural Science Foundation (2018CFB262) and Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (no. CUG170646) and a grant from the future R&D Program (2E28020) of Korea Institute of Science and Technology (KIST).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faheem, Du, J., Kim, S.H. et al. Application of biochar in advanced oxidation processes: supportive, adsorptive, and catalytic role. Environ Sci Pollut Res 27, 37286–37312 (2020). https://doi.org/10.1007/s11356-020-07612-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07612-y