Abstract
Microplastics (MPs) in natural environments have attracted lots of attention. Although the quantity of MPs present in terrene is much higher than that in aquatic environment, few studies have investigated the chemical behavior of MPs in terrestrial environment. This study investigate the Cu2+ (as a model heavy metal) adsorption capacity of six kinds of MPs (polyamide-6 (PA), polyethylene (PE), polystyrene (PS), polyethylene terephthalate (PET), polyvinyl chloride (PVC), polymethyl methacrylate (PMMA)) in batch adsorption experiments and the effects of different soil environmental factors, including pH and the presence of cations and low-molecular-weight organic acids (LMWOAs), as well as ultraviolet (UV) aging. The Cu2+ adsorption capacities of PA and PMMA were higher than those of other MPs and their maximum equilibrium adsorption capacities (estimated by the Langmuir adsorption equation) were 323.6 μg/g ± 38.2 and 41.03 ± 1.78 μg/g, respectively. The Cu2+ adsorption on MPs was affected by pH, and the greatest amount of Cu2+ adsorbed on PA and PMMA was observed at pH = 6 and pH = 7, respectively. The presence of Ca2+ or Mg2+ inhibited Cu2+ adsorption by MPs, due to competition for the adsorption sites. Moreover, Cu2+ adsorption by MPs was affected by various types of LMWOAs. The Cu2+ adsorption on PA was significantly reduced by citric acid, followed by oxalic acid, and oxalic acid was particularly evident for Cu2+ adsorption on PMMA. UV aging (200 h) had different effect on Cu2+ adsorption on MPs and it depends on the change of carbonyl index. Results demonstrate that soil environmental factors can change the ability of different MPs to adsorb Cu2+ and affect the transport of pollutants as carriers.
Similar content being viewed by others
Introduction
Plastic products are ubiquitous in our live, the annual production of plastics is drastically increasing, and it is expected to reach 33 billion tonnes by 2050 (Rochman et al., 2013). Plastics can be broken down through prolonged mechanical abrasion, ultraviolet (UV) radiation (Song et al., 2017), and microbial mineralization (Restrepo-Flórez et al., 2014) to form microplastics (MPs, size < 5 mm). MPs are widely spread across aquatic and terrestrial environments (Rios Mendoza and Balcer, 2018; He et al., 2018). As the main area where plastics are manufactured, terrestrial environments contain more MPs than aquatic environments. Previous studies reported that the annual amount of plastic released to land is 4–23 times higher than that released to oceans (Horton et al., 2017). Fuller and Gautam (2016) found that the concentrations of MPs widely varied (300–67,500 mg/kg) depend on the size in industrial soils from Sydney, Australia. Another study shows that average 55.5 mg/kg of MPs were found in 26 floodplain soil sites in Switzerland (Scheurer and Bigalke, 2018).
MPs are harmful to terrestrial life such as earthworms (Huerta-Lwanga et al., 2016; Rodriguez-Seijo et al., 2018), soil collembolans (Zhu et al., 2018), and other animals (Anbumani and Kakkar, 2018). In addition, MPs could reside in deeper soil or possibly arrive groundwater though transported by earthworms (Rillig et al., 2017) and pose severe threats to human health through their entry into foods such as salt (Yang et al., 2015), seafood (Karami et al., 2018), and drinking water (Mintenig et al., 2019). Apart from their inherent toxicity, MPs serve as carriers that can transport different kinds of pollutants due to their electric charge, high-specific surface area, and hydrophobicity (Zhu et al., 2019). They have the ability to adsorb and transfer hazardous chemicals to organisms, thereby increasing the harm of such pollutants (Wang et al., 2018a). The adsorption of organic pollutants by MPs has been widely reported (Guo et al., 2018; Wang et al., 2018c), and the adsorptio
n of heavy metals by MPs in the marine environment is gradually gaining attention (Ashton et al., 2010; Holmes et al., 2012). Previous research showed that toxicity due to Ni in combination with PS-COOH (polystyrene with a carboxyl group) is higher than that observed in the case of acute Ni toxicity alone (Kim et al., 2017). Another study on Zn bioavailability revealed that high-density polyethylene (HDPE) fragments can adsorb Zn, and MPs can increase Zn bioavailability (Hodson et al., 2017). Therefore, it is imperative to investigate the adsorption of heavy metals on MPs to understand the transportation and toxicities of heavy metals coupled with MPs.
Despite the presence of huge quantities of MPs in adsorbed pollutants of total environment, most previous studies on the adsorption of MPs were mainly conducted in relation to the aquatic environment. Some attempts have been made to investigate the adsorption of organic pollutants in the terrestrial environment (Liu et al., 2018). However, little attention has been paid to the adsorption of heavy metals on MPs in the soil and the effects of different soil environmental factors on heavy metals adsorption. The soil environment can be affected by several factors and the extent of changes in soil environmental factors, such as soil pH ranging from 3 to 8 and high concentrations of inorganic cations and low-molecular-weight organic acids (LMWOAs), is far beyond what is found in an aqueous environment. Therefore, it is imperative to investigate the adsorption of pollutants on MPs under different soil environmental conditions. In addition, UV aging results in further accelerate fragmentation of plastic into smaller particles (MPs) (Briassoulis et al., 2015a; Song et al., 2017), changes in the functional groups on the plastic surface (Kelkar, 2017), and higher adsorbance of pollutants.
Copper (Cu) is one of the most common heavy metals in the environment. The contents of total and availability (DTPA-extractable) of Cu are 15–40 mg/kg and 0.1–10 mg/kg in soil, respectively (Ni et al., 2003). Although Cu is a trace element necessary for life, excessive amounts are harmful to organisms in terrestrial environments (Wang et al., 2018b). Thus, it will be used to model heavy metals in this paper. Study of the adsorption mechanism of Cu2+ on MPs is vital as the foundation for the investigation of the compound pollution of MPs and heavy metals. The present study endeavored to investigate Cu2+ adsorption on MPs upon variation of various environmental factors including pH, the quantities of Ca2+, Mg2+, LMWOAs present, and UV aging. This study will highlight the important factors controlling Cu2+ adsorption on MPs, which will help to predict Cu2+ transport in the soil environment. In addition, the findings of this study will provide a basis for investigating the role of MPs as a carrier and transporter of heavy metals to soil organisms in the future.
Materials and methods
Materials
Six kinds of MPs (polyamide-6 (PA), polyethylene (PE), polystyrene (PS), polyethylene terephthalate (PET), polyvinyl chloride (PVC), and polymethyl methacrylate (PMMA)) were used in this study. PA and PVC were purchased from Aladdin Biochemical Polytron Technologies Inc. (Shanghai, China). PE, PET, and PS were purchased from Cheng Xinzhe Plastic Chemical Co. Ltd. (Suzhou, China). PMMA was purchased from Polymer Plastic Co. Ltd. (Dongguan, China). All chemical agents used were guaranteed reagent grade, and all solutions were prepared in high-purity Milli-Q water (Millipore Ltd., USA). The experimental vessel was soaked in 10% HNO3 solution for 24 h and then washed with deionized water before the experiments.
Batch adsorption experiments
Adsorption isotherms
Adsorption isotherm experiments were conducted by adding 0.500 g of different MP particles to 25 mL solutions of different Cu2+ concentrations (0.05, 0.1, 0.5, 1, 2, 5, and 10 mg/L) consisting of a Cu(NO3)2 solution with 0.01 mol/L NaNO3 as the background electrolyte in plastic centrifuge tubes. In order to better observe the adsorption characteristics, we selected the amount of microplastics higher than the environmental concentration. Based on the findings of kinetic experiments conducted in the present study (details in Fig. S1) and previous studies (Godjevargova and Mihova, 2003; Holmes et al., 2012), the adsorption time that the adsorption to reach equilibrium was identified as 24 h. After shaking at 200 rpm for 24 h, all samples were first centrifuged at 5000 rpm and then filtered through 0.45-μm polyethersulfone membrane filters (Germany). The control adsorption experiments containing Cu(NO3)2 and NaNO3 without MPs were also simultaneously conducted, and this concentration of Cu2+ was referred as initial concentration (preliminary experiments found that the recovery of Cu2+ in control is 96.0–102.5%). The amount of Cu2+ adsorbed by MPs was calculated by the mass balance method (the difference between control and other treatment concentrations) (Hodson et al., 2017). All experiments were performed in triplicate and the temperature was 25 °C. The filtered solution samples were acidified with 2% HNO3 to determine the Cu concentration.
The Cu2+ adsorption isotherms of the MPs were described by the Langmuir Eq. (1) and the Freundlich Eq. (2):
where Qm (μg/g) is the maximum adsorption capacity of the MP, Ce (μg/L) is the equilibrium concentration of the solute, and KL (L/g) is a Langmuir constant that characterizes the rate of the adsorption.
where KF (L/g) is the Freundlich adsorption coefficient, which relates to adsorption capacity, Ce (μg/L) is the equilibrium concentration of the solute, and b is the Freundlich isotherm exponent that indicates non-linearity.
Effects of pH, inorganic cations, and organic acids on the adsorption of Cu2+ by MPs
The effect of pH on the Cu2+ adsorption was examined by adjusting the pH of the solutions to 4–8 (at intervals of 1 unit) using NaOH or HNO3 solutions. To examine the influence of other inorganic cations on Cu2+ adsorption, Ca2+ and Mg2+ were added to the suspensions with final concentrations of 7.7 mM and 6.5 mM, respectively. The concentrations used were equivalent to the values of their soil concentrations (1.54% and 0.78%, respectively) (Wei et al., 1991). Citric acid (CA), oxalic acid (OA), tartaric acid (TA), and malic acid (MA) were used to examine the influence of LMWOAs on Cu2+ adsorption. The molar ratios of Cu2+ to LMWOAs were set at 1:0.25, 1:0.5, 1:1, 1:2, and 1:4. The pH of all solutions (except in the pH experiment) was controlled at 6 by adding NaOH or HNO3. 0.5 g of different MPs and 25 mL of solutions of different Cu2+ concentrations were added to plastic centrifuge tubes. The solution pH, inorganic cation concentration, and the LMWOA concentration were identified based on the above experimental design. After shaking at 200 rpm for 24 h, all samples were first centrifuged at 5000 rpm and then filtered through 0.45-μm polyethersulfone membrane filters. All filtered solution samples were acidified by HNO3 and each treatment was repeated in triplicate.
Effects of UV aging on Cu2+ adsorption by MPs
The aging of plastics is a very long process; however, UV irradiation can accelerate the aging process. MPs were exposed to UVA-340 nm fluorescent lamps in the customized UV Illumination incubator (SANYO MLR-351H, Japan), and the UV irradiance applied to those MPs was 15.7 W/m2, which was measured using a TM-213 light meter (TENMARS, Taiwan). MPs were aged for 200 h with the humidity and the temperature at 60% and 30 °C, respectively. Afterward, the aged MPs were used to carry out Cu2+ adsorption isotherm experiments as described in the “Adsorption isotherms” section.
Analytical methods
The morphology of six kinds of MPs was observed using a scanning electron microscope (SEM, Hitachi S-4800, Japan) and selected a representative figure to show, while the specific surface areas were investigated by N2/BET method (Autosorb-iQ, Quantachrome Instruments, USA). Attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectroscopy was used to analyze the MPs before the experiments. ATR-FTIR was carried out using OMNIC 8.0 (Thermo Nicolet, USA) to distinguish the surface groups via a Nicolet iS10 (USA) spectrophotometer. Zeta potentials of the MPs at pH 6 were determined using a zeta meter (Zetasizer Nano ZS90, Malvern Instruments Ltd., UK). Cu concentrations in the solutions were determined by a flame atomic absorption spectrophotometer (Hitachi Z-2000, Japan). The aging MPs were also analyzed by ATR-FTIR and SEM.
Statistical methods
Cu speciation in different pH (Cu(NO3)2 = 0.5 mg/L, pH = 4, 5, 6, 7, 8) and the presence of LMWOAs (add components Cu2+ = 0.5 mg/L, NO3− = 1.0 mg/L and different concentrations LMWOAs, temperature was set to 25 °C and pH fixed at 6, the molar ratios of Cu2+ to LMWOAs were set at 1:0.25, 1:0.5, 1:1, 1:2, and 1:4.) was analyzed by using Visual MINTEQ 3.1. Statistical analysis was performed using Excel 2010 (Microsoft, USA), SPSS 18.0 (IBM, USA), and comparisons among effects of inorganic cations treatments were performed by two independent one-way ANOVA and followed by Duncan’s test at the p < 0.05. All figures were drawn by Origin 2017 (OriginLab, USA).
Results and discussion
Characteristics of different kinds of MPs
The different MPs had distinct morphologies, and their characteristics are shown in Table 1. PA particles were porous and PMMA particles were spherical, while other materials were of irregular shape. The particle sizes of MPs ranged from 70 to 350 μm. The zeta potentials of different kinds of MPs were determined at pH 6, PA had the smallest zeta potential (− 7.59 mV), followed by PVC (− 8.70 mV), while the zeta potentials of other materials were relatively close (− 15.2 mV~− 16.1 mV). In contrast, the specific surface area of PA is much larger than other MPs, up to 8.71 m2/g, scanning electron microscopy confirmed that the surface of PA has many micro-pores, and the order of the surface areas was PA > PE > PVC > PET > PMMA > PS. Meanwhile, FTIR spectra revealed different MPs have different structures and properties (Fig. S2). PA has an amide group (–CONH–) that is chromogenic, is strongly polar, and is susceptible to the effects of UV, water, temperature, and other environmental factors (Lu et al., 2005). PE only has carbon–carbon bonds (–C–C–) and a long carbon chain, while PVC and PS include a chlorine atom and benzene rings among the carbon–carbon bonds, respectively. PET has benzene rings and an ester group (–COO–) in the long-chain polymers, and PMMA has an ester group (–COO–) on the branched chain. Sorption of contaminants on microplastics was related to the physicochemical properties of the polymer (Alimi et al., 2018).
Cu2+ adsorption on different kinds of MPs
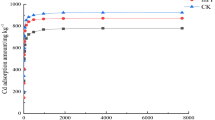
The Cu2+ adsorption capacities of PA and PMMA were significantly higher compared with the other MPs (PE, PS, PET, PVC) (Fig. 1) and the equations of Langmuir and Freundlich were used to fit the adsorption isotherms (Table 2). Cu2+ adsorption on PA, PE, PET, and PVC was better described by the Freundlich equation while the adsorption on PS and PMMA was better fitted the Langmuir equation. The maximum adsorption capacities of PA and PMMA derived from the Langmuir equation were 324 ± 38.2 μg/g and 41.0 ± 1.78 μg/g, respectively. However, the maximum adsorption capacities of PE, PS, PET, and PVC were < 10 μg/g.
Ashton et al. (2010) investigated the Cu2+ adsorption capacity of PE MPs in the coastal zones of south England and found that the Cu2+ adsorption capacity of original PE plastic granules was only 0.28 ± 0.18 μg/g. In another study, conducted at four locations in south-west England, Holmes et al. (2012) found that the capacities of microplastic pellets to adsorb Cu2+ ranged from 0.064 to 1.32 μg/g. Maybe salts in seawater could affect their adsorption. Overall, the adsorption capacities of heavy metals on MPs are related to their surface properties, such as their functional groups, specific surface area, and zeta potential. Brennecke et al. (2016) showed that the Cu2+ (released from antifouling paint to water) adsorption capacity of PVC (1320 μg/g) was much higher than that of PS (1100 μg/g) due to the higher specific surface area and the polar functional groups (chlorine) of PVC. Furthermore, the type of MP could play a more important role in the adsorption of contaminants than its other characteristics. In the present study, the higher adsorption capacities of PA and PMMA compared with the other MPs was associated with the polar functional groups (–NHCO– and –COO–) of PA and PMMA, which made them more hydrophilic and allowed more Cu2+ to adsorb on their surfaces. In addition, PA may also increase its adsorption capacity through the formation of hydrogen bonds (Li et al., 2018). The adsorption capacity of other materials was attributed to their zeta potential, specific surface area, and hydrogen bonding or polar interactions. Owing to PA and PMMA has stronger adsorption abilities; they were selected as representative MPs to study the influence of various environmental factors on Cu2+ adsorption.
Effects of pH and inorganic cations on Cu2+ adsorption by MPs
The Cu2+ adsorption capacity on PA reached the maximum when the pH was 6. However, other pH conditions had no significant influence on the adsorption capacity (Fig. 2a). That could be hydrophilic groups (–NHCO–) in both ends of the molecular chain make the solution stable under acid-base conditions. Cu2+ adsorption by PMMA significant increased from pH 4 to 7 and then decreased with further increases in pH, the maximum adsorption amount was 13.8 ± 0.401 μg/g. The H+ could compete with the Cu2+ for the adsorption site of PMMA, leading to a lower Cu2+ adsorption capacity when the pH is decreased. Our findings of a continuous increase in Cu2+ adsorption capacity for PMMA with increasing pH were in agreement with the findings of Demirata-Öztürk (1996), who also found that the Cu2+ adsorption capacity of PMMA increased when the pH increased from 4 to 7. The simulation of Cu morphology using Visual MINTEQ 3.1 showed that only Cu2+ and CuNO3+ exist in an acid solution (pH 4 and 5) (Fig. S3). When the pH ≥ 6, other dissolved Cu moieties (CuOH+, Cu2(OH)22+) begin to appear; when the pH > 7, the Cu2+ concentration could drop rapidly; at a pH of 8, Cu(OH)2 precipitate appears. The Cu2+ concentration is constantly reduced with increasing pH.
The presence of inorganic cations is deemed to affect Cu2+ adsorption on MPs. Ca2+ and Mg2+ are important cations in aquatic and terrestrial environments, and they affect the migration and adsorption of pollutants in both environments. As shown in Fig. 2b, in the presence of Ca2+ and Mg2+ (the concentrations of Ca2+ and Mg2+ all matched environmental concentrations and were higher than the Cu2+ concentration), Cu2+ adsorption on MPs was inhibited. In addition, Cu2+ adsorption on PMMA was more easily affected by Ca2+ and Mg2+ than that on PA, and Ca2+ had a greater inhibitory effect on Cu2+ adsorption than Mg2+. When Ca2+ and Mg2+ exist, the amount of Cu2+ adsorbed by PMMA were 5.80 ± 0.568 μg/g and 6.93 ± 2.05 μg/g, compare with blank (13.2 ± 1.14 μg/g) (the amount of Cu2+ adsorbed by PA in the treatment of blank, Ca2+, Mg2+ was 22.3 ± 0.328, 18.6 ± 0.328, 22.2 ± 0.000 μg/g). These cations compete with Cu2+ at the adsorption sites of the MP surface, which will in turn affect the Cu2+ adsorption. For instance, for PA modified with immobilized dead yeast cells, high concentrations of Ca2+ and Mg2+ reduced Cu2+adsorption (Godjevargova and Mihova, 2003). It was also found that Ca2+ was more influential than Mg2+ when the concentrations of Ca2+ and Mg2+ reached 2000 mg/L, and the adsorption capacities of Cu2+ decreased by more than 50% and 10%, respectively.
Effects of organic acids on Cu2+ adsorption by MPs
Low-molecular-weight organic acids (LMWOAs) can complex with heavy metals in the soil and thus affect their behavior in the soil environment (Yang et al., 2006). Four common LMWOAs (citric acid, oxalic acid, tartaric acid, and malic acid) were selected as representatives to assess the effects of organic acids on Cu2+ adsorption by MPs. Cu2+ adsorption by PA was significantly reduced in the presence of citric acid, followed by oxalic acid, while no substantial effects of tartaric acid and malic acid on Cu2+ adsorption were observed (Fig. 3). The capacity of organic acids to chelate with Cu2+ is positively correlated with log K (log K is complex stability constant), and Wang et al. (2009) found that the stronger the coordination of an organic acid with Cu2+, the greater the decrease in Cu2+ adsorption onto the Hydroxyapatite. The log K values (25 °C, ionic strength = 0.1 M) of the organic ligands with Cu follow the order: citric acid > oxalic acid > malic acid > tartaric acid (Martell and Smith, 1977). Oxalic acid exhibited an obvious inhibiting effect on Cu2+ adsorption by PMMA. In contrast, low and high concentrations of citric acid (mole ratios of Cu citric acid > 1 and < 1) promoted and inhibited Cu2+ adsorption by PMMA, respectively. Citric acid could provide more adsorption sites for PMMA to adsorb Cu2+ when it adsorbed on PMMA. These results were similar with Cu2+ adsorption on biochar effected by citric acid and oxalic acid (Zhou et al., 2016). From Fig. 4a, when the mole ratios of Cu:citric acid > 1, the main Cu fraction was Cu2+ (positive charged), which was conductive to the Cu adsorption on PMMA. However, when the mole ratios of Cu:citric acid < 1, the main Cu fraction was Cu-citrate− (negative charged), which was not conductive to the Cu adsorption on PMMA. Citric acid and oxalic acid are strong complexing agents for Cu2+. The proportion of Cu2+ significantly decreased with increasing concentration of citric acid and oxalic acid. In contrast, tartaric acid and malic acid exhibited much weaker complexing abilities towards Cu2+ (Fig. 4).
It is possible that competitive adsorption sites on the plastic surface can reduce the adsorption of Cu2+ on the surface of MPs. Furthermore, low concentrations of citric acid can be used as complexing bridges to enhance the affinity of small-molecular organic acids for the MP surface, thus increasing Cu2+ adsorption on the surface of the MPs. Previous studies also revealed that organic acids can increase Cu2+ adsorption on the surface of hydroxyapatite and soil (Hu, 2005; Wang et al., 2009). Consequently, LMWOAs affect the Cu2+ adsorption of MPs is via the complexation of it with Cu2+. The presence of oxalic acid reduces the adsorbed Cu2+ on MPs, adsorption of Cu is almost not affected by tartaric acid and malic acid. In addition, the effect of citric acid on adsorption depends on MPs.
Effects of UV aging on Cu2+ adsorption by MPs
Figure 5 and Table S1 showed that Cu2+ adsorption on PA slightly decreased while it slightly increased on PMMA after UV aging. The adsorption capacities on aged PA and PMMA were 265 ± 25.7 μg/g and 79.4 ± 10.5 μg/g, respectively. ATR-FTIR spectra show that the absorption peaks of aged MPs are lower than those of the virgin MPs (Fig. S4), arising from the decomposition of the –C–C– chain, which could reduce molecular weights (Pushpadass et al., 2010). The carbonyl index of the spectra was defined as the ratio of the carbonyl absorption band to the optical density of the methylene absorption band. The carbonyl indexes could be obtained by calculating the magnitudes of the absorption peaks of virgin and aged MPs (Yousif et al., 2012; Briassoulis et al., 2015b). The absorption peaks of the carbonyl group and reference alkyl group in PA and PMMA were at 1634.9 cm−1 and 1439.6 cm−1 and 1721.2 cm−1 and 1434.3 cm−1, respectively (Table 3). The carbonyl index of PA decreased from 13.6 to 9.04 while it increased from 2.79 to 4.94 for PMMA after UV aging. Müller et al. (2018) have found that PP (polypropylene) and PS weathered in UVA-340 nm fluorescent lamps (UV irradiance was 26 W/m2) at 60 °C for 4 weeks. Carbonyl bands increased from 0.22 to 1.65 and from 0.33 to 1.24 (area unit), respectively. Hüffer et al. (2018) also found that UVC weathered PS MPs for 96 h make the carbonyl index increased from 0.36 to 1.61. (UVA and UVC are two types of ultraviolet light; UVA (320–420 nm) is generally used for aging test; UVC (200–275) is short-wave ultraviolet light is mainly used for sterilization.) The positive correlation between the Cu2+ adsorption capacities of the two MPs after aging and the number of carbonyl groups was consistent with previous studies. Godjevargova and Mihova (2003) have found that modified PA beads have more carbonyl groups than unmodified PA beads, and this makes the former adsorb more Cu2+.
It has been previously reported that many types of polymers are susceptible to UV degradation because the energy of UV radiation exceeds that of all the strongest carbon bonds (Murray et al., 2012). UV light can cause fracture of the polymer, a decrease in molecular weight, and the production of free radicals, or it may cause an increase in the length of the molecular chain and the crosslinking of the polymer (Yousif and Haddad, 2013). Different materials may require different aging conditions. Li et al. (2008) reported that carboxyl groups appeared in pure polypropylene after exposure for 80 days outdoors in Beijing, China. Degradation occurred and carbonyl peaks appeared after low-density polyethylene (LDPE) was exposed outdoors for 3000 h in Messina, Sicily (Italy) (Severini et al., 1993). Wavelengths less than 300 nm could lead to the generation of amide groups and breakage of N–C bonds in PA, while wavelengths ranging from 340 to 400 nm could lead to the production of free radicals in the polymer (Hu, 1998). The degradation of plastics might be affected by physical, chemical, and biological aspects of the comprehensive factors present under natural conditions, and cause an increase in the adsorption quantity when the number of oxygen functional groups increases. Generally, the adsorption capacity of aged MPs in the environment is greater than that of virgin MPs (Holmes et al., 2012; Mato et al., 2001; Turner and Holmes, 2015). The aging conditions need to be further optimized to determine the aging effects on the adsorption of heavy metals by MPs.
Conclusions
The results of this study reveal Cu2+ adsorption is greatly influenced by the type of MP. The Cu2+ adsorption capacities of PA and PMMA were higher than those of the other MPs (PE, PS, PET, PVC) and were affected by the surface area, chemical group, etc. The Cu2+ adsorption capacities of PA and PMMA reach the maximum when pH is at 6 and 7, respectively. The presence of Ca2+ and Mg2+ inhibited Cu2+ adsorption by MPs. Different concentrations and types of LMWOAs had different effects on Cu2+ adsorption. The presence of oxalic acid reduces the adsorbed Cu2+ on MPs, adsorption of Cu is almost not affected by tartaric acid and malic acid. In addition, the effect of citric acid on adsorption depends on MPs. In addition, UV aging (200 h) had a different effect on Cu2+ adsorption by MPs, which depends on the change of carbonyl index. Overall, different soil environmental factors could affect Cu2+ adsorption on MPs, and this provides important data that improve our understanding of the behavior of MPs in a terrestrial environment. Further studies are required and the focus should include (1) how different components of the soil compete with MPs to adsorb Cu2+, (2) investigation of the adsorption capacity of aged MPs, and (3) investigation of the adsorption of other heavy metals on MPs in a terrestrial environment.
References
Alimi OS, Farner Budarz J, Hernandez LM, Tufenkji N (2018) Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ Sci Technol 52(4):1704–1724. https://doi.org/10.1021/acs.est.7b05559
Anbumani S, Kakkar P (2018) Ecotoxicological effects of microplastics on biota: a review. Environ Sci & Pollut R 25:14373–14396. https://doi.org/10.10007/s11356-018-1999-x
Ashton K, Holmes L, Turner A (2010) Association of metals with plastic production pellets in the marine environment. Mar Pollut Bull 60(11):2050–2055. https://doi.org/10.1016/j.marpolbul.2010.07.014
Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J (2016) Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci 178:189–195. https://doi.org/10.1016/j.ecss.2015.12.003
Briassoulis D, Babou E, Hiskakis M (2015a) Degradation in soil behavior of artificially aged polyethylene films with pro-oxidants. J Appl Polym Sci 132(30):3262–3271. https://doi.org/10.1002/app.42289
Briassoulis D, Babou E, Hiskakis M (2015b) Analysis of long-term degradation behaviour of polyethylene mulching films with pro-oxidants under real cultivation and soil burial conditions. Environ Sci Pollut Res 22(4):2584–2598. https://doi.org/10.1007/s11356-014-3464-9
Demirata-Öztürk B, Gümüs G, Öncül-Koc A, Catalgil-Giz H (1996) Preconcentration of copper ion in aqueous phase on methacrylate polymers. J Appl Polym Sci 62(4):613–616. https://doi.org/10.1002/(SICI)1097-4628(19961024)62:4<613::AID-APP3>3.0.CO;2-W
Fuller S, Gautam A (2016) A procedure for measuring microplastics using pressurized fluid extraction. Environ Sci Technol 50(11):5774–5780. https://doi.org/10.1021/acs.est.6b00816
Godjevargova T, Mihova S (2003) Adsorption of copper on specifically modified polyamide sorbent. J Appl Polym Sci 90(1):80–85. https://doi.org/10.1002/app.12539
Guo XT, Pang JW, Chen SY, Jia HZ (2018) Sorption properties of tylosin on four different microplastics. Chemosphere 209:240–245. https://doi.org/10.1016/j.chemosphere.2018.06.100
He DF, Luo YM, Lu SB, Liu MT, Song Y, Lei LL (2018) Microplastics in soils: analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal Chem 109:163–172. https://doi.org/10.1016/j.trac.2018.10.006
Hodson ME, Duffus-Hodson CA, Clark A, Prendergast-Miller MT, Thorpe KL (2017) Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ Sci Technol 51(8):4714–4721. https://doi.org/10.1021/acs.est.7b00635
Holmes LA, Turner A, Thompson RC (2012) Adsorption of trace metals to plastic resin pellets in the marine environment. Environ Pollut 160(1):42–48. https://doi.org/10.1016/j.envpol.2011.08.052
Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C (2017) Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ 586:127–141. https://doi.org/10.1016/j.scitotenv.2017.01.190
Hu H (2005) Effects of several organic acids on copper adsorption by soils with permanent and variable charges. Acta Pedol Sin 42(2):232–237. (in Chinese). https://doi.org/10.3321/j.issn:0564-3929.2005.02.009
Hu X (1998) Wavelength sensitivity of photo-oxidation of polyamide 6. Polym Degrad Stab 62(3):599–601. https://doi.org/10.1016/S0141-3910(98)00046-9
Huerta-Lwanga E, Gertsen H, Gooren H, Peters P, Salanki T, van der Ploeg M, Besseling E, Koelmans AA, Geissen V (2016) Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ Sci Technol 50(5):2685–2691. https://doi.org/10.1021/acs.est.5b05478
Hüffer T, Weniger AK, Hofmann T (2018) Sorption of organic compounds by aged polystyrene microplastic particles. Environ Pollut 236:218–225. https://doi.org/10.1016/j.envpol.2018.01.022
Karami A, Golieskardi A, Choo CK, Larat V, Karbalaei S, Salamatinia B (2018) Microplastic and mesoplastic contamination in canned sardines and sprats. Sci Total Environ 612:1380–1386. https://doi.org/10.1016/j.scitotenv.2017.09.005
Kelkar V (2017) Analysis of chlorination & UV effects on microplastics using Raman spectroscopy. Arizona state university, Tempe, pp 18–19
Kim D, Chae Y, An YJ (2017) Mixture toxicity of nickel and microplastics with different functional groups on Daphnia magna. Environ Sci Technol 51(21):12852–12858. https://doi.org/10.1021/acs.est.7b03732
Li J, Yang R, Yu J, Liu Y (2008) Natural photo-aging degradation of polypropylene nanocomposites. Polym Degrad Stab 93(1):84–89. https://doi.org/10.1016/j.polymdegradstab.2007.10.022
Li J, Zhang K, Zhang H (2018) Adsorption of antibiotics on microplastics. Environ Pollut 237:460–467. https://doi.org/10.1016/j.envpol.2018.02.050
Liu J, Ma Y, Zhu D, Xia T, Qi Y, Yao Y, Guo X, Ji R, Chen W (2018) Polystyrene Nanoplastics-enhanced contaminant transport: role of irreversible adsorption in glassy polymeric domain. Environ Sci Technol 52(5):2677–2685. https://doi.org/10.1021/acs.est.7b05211
Lu G, Zhu H, Lin A, Gan F (2005) Recent development in weathering study of polyamide. Equip Environ Eng 2:41–46. https://doi.org/10.3969/j.issn.1672-9242.2005.03.009
Martell AE, Smith RM (1977) Other organic ligands. Critical stability constants. 3. Plenum press, New York
Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ Sci Technol 35:318–324. https://doi.org/10.1021/es0010498
Mintenig SM, Loder MGJ, Primpke S, Gerdts G (2019) Low numbers of microplastics detected in drinking water from ground water sources. Sci Total Environ 648:631–635. https://doi.org/10.1016/j.scitotenv.2018.08.178
Müller A, Becker R, Dorgerloh U, Simon FG, Braun U (2018) The effect of polymer aging on the uptake of fuel aromatics and ethers by microplastics. Environ Pollut 240:639–646. https://doi.org/10.1016/j.envpol.2018.04.127
Murray MP, Bruckman LS, French RH (2012) Photodegradation in a stress and response framework: poly(methyl methacrylate) for solar mirrors and lens. J Photonics Energy 2(1):022004. https://doi.org/10.1117/1.JPE.2.022004
Ni WZ, Ma HY, Yu S, He JX (2003) Copper pollution in soil-plant systems and its ecological and health effects. Guangdong Trace Elem Sci 10(1):1–5. https://doi.org/10.16755/j.cnki.issn.1006-446x.2003.01.001
Pushpadass HA, Bhandari P, Hanna MA (2010) Effects of LDPE and glycerol contents and compounding on the microstructure and properties of starch composite films. Carbohydr Polym 82(4):1082–1089. https://doi.org/10.1016/j.carbpol.2010.06.032
Restrepo-Flórez JM, Bassi A, Thompson MR (2014) Microbial degradation and deterioration of polyethylene – a review. Int Biodeterior Biodegrad 88:83–90. https://doi.org/10.1016/j.ibiod.2013.12.014
Rodriguez-Seijo A, da Costa JP, Rocha-Santos T (2018) Oxidative stress, energy metabolism and molecular responsed of earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics. Environ Sci & Pollut R 25:33599–33610. https://doi.org/10.1007/s11356-018-3317-z
Rillig MC, Ziersch L, Hempel S (2017) Microplastic transport in soil by earthworms. Sci Rep 7:1362. https://doi.org/10.1038/s41598-017-01594-7
Rios Mendoza LM, Balcer M (2018) Microplastics in freshwater environments: a review of quantification assessment. TrAC Trends Anal Chem 113:402–408. https://doi.org/10.1016/j.trac.2018.10.020
Rochman CM, Hoh E, Kurobe T, Teh SJ (2013) Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci Rep 3:3263. https://doi.org/10.1038/srep03263
Scheurer M, Bigalke M (2018) Microplastics in Swiss floodplain soils. Environ Sci Technol 52(6):3591–3598. https://doi.org/10.1021/acs.est.7b06003
Severini F, Gallo R, Ipsale S, Ricca G (1993) Environmental degradation of LDPE observed by UV spectroscopy. Polym Degrad Stab 41(1):103–107. https://doi.org/10.1016/0141-3910(93)90068-T
Song YK, Hong SH, Jang M (2017) Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ Sci Technol 51(8):4368–4376. https://doi.org/10.1021/acs.est.6b06155
Turner A, Holmes LA (2015) Adsorption of trace metals by microplastic pellets in fresh water. Environ Chem 12(5):600–610. https://doi.org/10.1071/EN14143
Wang YJ, Chen JH, Cui YX, Wang SQ, Zhou DM (2009) Effects of low-molecular-weight organic acids on Cu(II) adsorption onto hydroxyapatite nanoparticles. J Hazard Mater 162(2–3):1135–1140. https://doi.org/10.1016/j.jhazmat.2008.06.001
Wang F, Wong CS, Chen D, Lu XW, Wang F, Zeng EY (2018a) Interaction of toxic chemicals with microplastics: a critical review. Water Res 139:208–219. https://doi.org/10.1016/j.watres.2018.04.003
Wang M, Li SS, Li XY, Zhao ZQ, Chen SB (2018b) An overview of current status of cupper pollution in soil and remediation efforts in China. Earth Sci Front 25(5):305–313. (in Chinese). https://doi.org/10.13745/j.esf.sf.2018.4.20
Wang W, Wang J (2018c) Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics. Chemosphere 193:567–573. https://doi.org/10.1016/j.chemosphere.2017.11.078
Wei FS, Chen JS, Wu YY, Zheng CJ (1991) Study on the Background contents on element of soils in China. Chin J Environ Sci 12(4):12–19. https://doi.org/10.13227/j.hjkx.1991.04.005
Yang D, Shi H, Li L, Li J, Jabeen K, Kolandhasamy P (2015) Microplastic pollution in table salts from China. Environ Sci Technol 49(22):13622–13627. https://doi.org/10.1021/acs.est.5b03163
Yang JY, Yang XE, He ZL, Li TQ, Shentu JL, Stoffella PJ (2006) Effects of pH, organic acids, and inorganic ions on lead desorption from soils. Environ Pollut 143(1):9–15. https://doi.org/10.1016/j.envpol.2005.11.010
Yousif E, Salimon J, Salih N, Ahmed A (2012) Improvement of the photostabilization of PMMA films in the presence 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole. Journal of King Saud University – Sci 24(2):131–137. https://doi.org/10.1016/j.jksus.2010.09.001
Yousif E, Haddad R (2013) Photodegradation and photostabilization of polymers, especially polystyrene: review. SpringerPlus 2(1):1–32. https://doi.org/10.1186/2193-1801-2-398
Zhou DD, Liang N, Li H, Zhang D, Wu M, Pan B (2016) Effect of low molecular weight organic acids on Cu(II)adsorption by biochars. J. Agro-Environ. Sci 35(10):1923–1930. https://doi.org/10.11654/jaes.2016-0376
Zhu D, Chen QL, An XL (2018) Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol Biochem 116:302–310. https://doi.org/10.1016/j.soilbio.2017.10.027
Zhu YG, Zhu D, Xu T, Ma J (2019) Impacts of (micro)plastics on soil ecosystem: Progress and perspective. J Agro-Environ Sci 38(1):1–6. https://doi.org/10.11654/jaes.2018-1427
Funding
This research was financially supported by the Natural Science Foundation of China (41471261, 21177135).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All experiments comply with the current laws of China. This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1346 kb)
Rights and permissions
About this article
Cite this article
Yang, J., Cang, L., Sun, Q. et al. Effects of soil environmental factors and UV aging on Cu2+ adsorption on microplastics. Environ Sci Pollut Res 26, 23027–23036 (2019). https://doi.org/10.1007/s11356-019-05643-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05643-8