Abstract
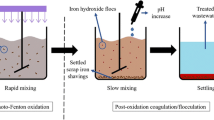
Multistage Fenton oxidation is a favored method for the treatment of benzene dye intermediate (BDI) wastewater, but the pH adjustments required after each stage of the Fenton process with a simple way is still a challenge. Limestone pretreatment and lime neutralization-coagulation were used to solve the problem in multistage Fenton process. First, we determined the optimal conditions of Fenton oxidation using the Box-Behnken response surface method. Limestone pretreatment before the multistage Fenton process allowed for simultaneous pH adjustment and 14.15% COD removal. Most notably, the lime cream neutralization-coagulation process effectively adjusted the pH after each stage of the Fenton process. The optimum CaO particle size, lime mass fraction, mixing time, and stirring speed were determined by orthogonal tests. COD removal (89.23%) was obtained when lime cream neutralization-coagulation was applied to the three-staged Fenton process, while only 58.57% COD removal was obtained by the unadjusted single-staged Fenton process. The COD and wastewater color were reduced from 10,600 mg/L and 12,200 multiples to 495 mg/L and 20 multiples, respectively, using the adjusted process. This improved method provides a promising cost-effective way to efficiently treat real BDI wastewater.








Similar content being viewed by others
References
Babuponnusami A, Muthukumar K (2014) A review on Fenton and improvements to the Fenton process for wastewater treatment. J Environ Chem Eng 2:557–572
Behin J, Akbari A, Mahmoudi M, Khajeh M (2017) Sodium hypochlorite as an alternative to hydrogen peroxide in Fenton process for industrial scale. Water Res 121:120–128
Boukhoubza F, Jail A, Korchi F, Idrissi LL, Hannache H, Duarte JC, Hassani L, Nejmeddine A (2009) Application of lime and calcium hypochlorite in the dephenolisation and discolouration of olive mill wastewater. J Environ Manag 91:124–132
Bouzayani B, Bocos E, Elaoud SC, Pazos M, Sanromán MÁ, González-Romero E (2017) An effective electroanalytical approach for the monitoring of electroactive dyes and intermediate products formed in electro-Fenton treatment. J Electroanal Chem 808:403–411
Carra I, López JLC, Santos-Juanes L, Malato S, Pérez JAS (2013) Iron dosage as a strategy to operate the photo-Fenton process at initial neutral pH. Chem Eng J 224:67–74
Chang MW, Chern JM (2010) Decolorization of peach red azo dye, HF6 by Fenton reaction: initial rate analysis. J Taiwan Inst Chem Eng 41:221–228
Cheng Y, Chen Y, Lu J, Nie J, Liu Y (2018) Fenton treatment of bio-treated fermentation-based pharmaceutical wastewater: removal and conversion of organic pollutants as well as estimation of operational costs. Environ Sci Pollut Res Int 25:1–13
Clescerl LS, Greenberg AE, Eaton AD (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Cong L, Zhou D, Wang J (2016) Removal of multi-dye wastewater by the novel integrated adsorption and Fenton oxidation process in a fluidized bed reactor. Environ Sci Pollut Res 23:1–11
Csd R, Rac B, Lima VN, Madeira LM (2018) p-Nitrophenol degradation by Fenton's oxidation in a bubble column reactor. J Environ Manag 206:774–785
De LJ, Truong LG, Legube B (2004) A comparative study of the effects of chloride, sulfate and nitrate ions on the rates of decomposition of H2O2 and organic compounds by Fe(II)/H2O2 and Fe(III)/H2O2. Chemosphere 55:715–723
Duesterberg CK, Waite TD (2006) Process optimization of Fenton oxidation using kinetic modeling. Environ Sci Technol 40:4189–4195
Fischbacher A, Von SC, Schmidt TC (2017) Hydroxyl radical yields in the Fenton process under various pH, ligand concentrations and hydrogen peroxide/Fe(II) ratios. Chemosphere 182:738–744
Georgiou D, Aivazidis A, Hatiras J, Gimouhopoulos K (2003) Treatment of cotton textile wastewater using lime and ferrous sulfate. Water Res 37:2248–2250
Ghadiri M, Shirazian S (2017) Numerical simulation of reactive extraction of benzoic acid from wastewater via membrane contactors. Environ Sci Pollut Res 24:1–10
Giannakis S, Jovic M, Gasilova N, Pastor Gelabert M, Schindelholz S, Furbringer JM, Girault H, Pulgarin C (2016) Iohexol degradation in wastewater and urine by UV-based advanced oxidation processes (AOPs): process modeling and by-products identification. J Environ Manag 195:174–185
Gotoh Y, Iwata G, Choh K, Kubota M, Matsuda H (2011) Trichloroethylene decomposition and in-situ dry sorption of Cl-products by calcium oxides prepared from hydrated limes. Chemosphere 85:637–642
Gu L, Zhu N, Wang L, Bing X, Chen X (2011) Combined humic acid adsorption and enhanced Fenton processes for the treatment of naphthalene dye intermediate wastewater. J Hazard Mater 198:232–240
Guo Y, Xue Q, Zhang H, Wang N, Chang S, Wang H, Pang H, Chen H (2018) Treatment of real benzene dye intermediates wastewater by the Fenton method: characteristics and multi-response optimization. Rsc Adv 8:80–90
Guo Y, Xue Q, Cui K, Zhang J, Wang H, Zhang H, Yuan F, Chen H (2018) Study on the degradation mechanism and pathway of benzene dye intermediate 4-methoxy-2-nitroaniline via multiple methods in Fenton oxidation process. RSC Adv 8:10764–10775
Hasani ZM, Alavi Moghaddam MR, Maknoon R (2015) Operation of integrated sequencing batch membrane bioreactor treating dye-containing wastewater at different SRTs: study of overall performance and fouling behavior. Environ Sci Pollut Res 22:5931–5942
Hsueh CC, You LP, Li JY, Chen CT, Wu CC, Chen BY (2016) Feasibility study of reduction of nitroaromatic compounds using indigenous azo dye-decolorizers. J Taiwan Inst Chem Eng 64:180–188
Jung G, Kim HI (2014) Synthesis and photocatalytic performance of PVA/TiO2/graphene-MWCNT nanocomposites for dye removal. J Appl Polym Sci 131:8797–8803
Kabra AN, Khandare RV, Govindwar SP (2013) Development of a bioreactor for remediation of textile effluent and dye mixture: a plant–bacterial synergistic strategy. Water Res 47:1035–1048
Lau YY, Wong YS, Ong SA, Ho LN, Hussin K, Lutpi NA (2017) Intermolecular mechanistic treatment of recalcitrant environmental pollutants: azo, benzene, naphthalene and vinyl sulfone. J Taiwan Inst Chem Eng 76:27–34
Lindsey ME, Xu G, Lu J, Tarr MA (2003) Enhanced Fenton degradation of hydrophobic organics by simultaneous iron and pollutant complexation with cyclodextrins. Sci Total Environ 307:215–229
Liu H, Wang C, Li X, Xuan X, Jiang C, Cui H (2007) A novel electro-Fenton process for water treatment: reaction-controlled pH adjustment and performance assessment. Environ Sci Technol 41:2937–2942
Long Y, Jing X, Shen D, Yao D, Feng H (2017) Effective removal of contaminants in landfill leachate membrane concentrates by coagulation. Chemosphere 167:512–519
Lu H, Zhou J, Wang J, Ai H, Zheng C, Yang Y (2008) Decolorization of anthraquinone dye intermediate and its accelerating effect on reduction of azo acid dyes by Sphingomonas xenophaga in anaerobic–aerobic process. Biodegradation 19:643–650
Luna MDGD, Veciana ML, Colades JI, Su CC, Lu MC (2014) Factors that influence degradation of acetaminophen by Fenton processes. J Taiwan Inst Chem Eng 45:565–570
Masomboon N, Ratanatamskul C, Lu MC (2009) Chemical oxidation of 2,6-dimethylaniline in the Fenton process. Environ Sci Technol 43:8629–8634
Moosvi S, Kher X, Madamwar D (2007) Isolation, characterization and decolorization of textile dyes by a mixed bacterial consortium JW-2. Dyes Pigments 74:723–729
Nam K, Rodriguez W, Kukor JJ (2001) Enhanced degradation of polycyclic aromatic hydrocarbons by biodegradation combined with a modified Fenton reaction. Chemosphere 45:11–20
Neyens E, Baeyens J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater 98:33–50
Nidheesh PV, Gandhimathi R, Ramesh ST (2013) Degradation of dyes from aqueous solution by Fenton processes: a review. Environ Sci Pollut Res 20:2099–2132
O’Coinceanainn M, Bonnely S, Baderschneider B, Hynes MJ (2004) Reaction of iron(III) with theaflavin: complexation and oxidative products. J Inorg Biochem 98:657–663
Pandit P, Basu S (2004) Removal of ionic dyes from water by solvent extraction using reverse micelles. Environ Sci Technol 38:2435–2442
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36:1–84
Romero V, Acevedo S, Marco P, Giménez J, Esplugas S (2015) Enhancement of Fenton and photo-Fenton processes at initial circumneutral pH for the degradation of the β-blocker metoprolol. Water Res 88:449–457
Rosli MM, Patil PS, Fun HK, Razak IA, Dharmaprakash SM (2007) 4-Methoxy-2-nitroaniline. Acta Crystallogr 63:1039–1040
Schenone AV, Conte LO, Botta MA, Alfano OM (2015) Modeling and optimization of photo-Fenton degradation of 2,4-D using ferrioxalate complex and response surface methodology (RSM). J Environ Manag 155:177–183
Subramanian G, Madras G (2016) Introducing saccharic acid as an efficient iron chelate to enhance photo-Fenton degradation of organic contaminants. Water Res 104:168–177
Wu YY, Zhou SQ, Qin FH, Peng HP, Lai YL, Lin YM (2010) Removal of humic substances from landfill leachate by Fenton oxidation and coagulation. Process Saf Environ Prot 88:276–284
Yu W, Yang J, Shi Y, Song J, Shi Y, Xiao J, Li C, Xu X, He S, Liang S, Wu X, Hu J (2016) Roles of iron species and pH optimization on sewage sludge conditioning with Fenton's reagent and lime. Water Res 95:124–133
Zhang H, Choi HJ, Huang CP (2005) Optimization of Fenton process for the treatment of landfill leachate. J Hazard Mater 125:166–174
Funding
This study was financially supported by the Fundamental Research Fund for the Central Universities (No. 2652017166), the fund of water quality control principles for safe storage of reclaimed groundwater (Project No. 51238001), the National Natural Science Foundation of China (Grant 41672239 and Grant 41572229), the Research Fund of China Geological Survey (1212011121166 and DD20160300), and the National Water Pollution Control and Treatment Science and Technology Major Project (2015ZX07406005-001).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Vítor Pais Vilar
Rights and permissions
About this article
Cite this article
Guo, Y., Xue, Q., Zhang, H. et al. Highly efficient treatment of real benzene dye intermediate wastewater by simple limestone and lime neutralization-coagulation with improved Fenton oxidation. Environ Sci Pollut Res 25, 31125–31135 (2018). https://doi.org/10.1007/s11356-018-3101-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3101-0