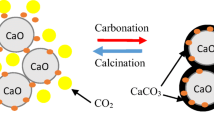
Abstract
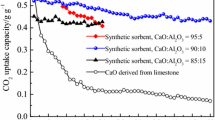
Using aluminum nitrate (AlN) and bauxite tailings (BTs) as different dopants, and lime mud (LM) as calcium source, a series of CaO-based sorbents were prepared for CO2 capture by dry mixing method; then, the carbonation conversions of multiple carbonation/calcination cycles were detected in a thermogravimetric analyzer (TGA). Effects of different dopants, dopant contents, precalcination conditions, and a long series of cycles on CO2 absorption properties were scrutinized, and the phase composition and morphologies were tested by scanning electron microscopy (SEM) and X-ray diffraction (XRD). Durability studies show that the sample doped with AlN remains a higher absorption conversion (30.88%) after 30 carbonation/calcination cycles. In the meantime, the sorbent doped with BTs showed a lower conversion, which is probably resulted from the impurities from waste BTs. However, the sample BT has a better cyclic absorption stability. In addition, the incorporation of BTs, as a kind of solid waste, not only decreases the preparation cost but also is good for environment. The occurrence of Ca12Al14O33 phase is considered to provide a stable framework inhibiting inactivation of CaO, and improve the CO2 adsorption stability.

ᅟ









Similar content being viewed by others
References
Al-Maythalony BA, Shekhah O, Swaidan R, Belmabkhout Y, Pinnau I, Eddaoudi M (2015) Quest for anionic MOF membranes: continuous sod-ZMOF membrane with CO2 adsorption-driven selectivity. J Am Chem Soc 137(5):1754–1757
Anbia M, Hoseini V (2012) Development of MWCNT@MIL-101 hybrid composite with enhanced adsorption capacity for carbon dioxide. Chem Eng J 191:326–330
Belaissaoui B, Willson D, Favre E (2012) Membrane gas separations and post-combustion carbon dioxide capture: parametric sensitivity and process integration strategies. Chem Eng J 211-212:122–132
Borgwardt RH (1989) Calcium oxide sintering in atmospheres containing water and carbon dioxide. Ind Eng Chem Res 28(4):493–500
Broda M, Müller CR (2012) Synthesis of highly efficient, ca-based, Al2O3-stabilized, carbon gel-templated CO2 sorbents. Adv Mater 24(22):3059–3064
Chen Z, Song HS, Portillo M, Lim CJ, Grace JR, Anthony EJ (2009) Long-term calcination/carbonation cycling and thermal pretreatment for CO2 capture by limestone and dolomite. Energy Fuel 23(3):1437–1444
Chen H, Zhao C, Yu W (2013) Calcium-based sorbent doped with attapulgite for CO2 capture. Appl Energy 112:67–74
Chen H, Wang F, Zhao C, Khalili N (2017) The effect of fly ash on reactivity of calcium based sorbents for CO2 capture. Chem Eng J 309:725–737
Chu F, Jon C, Yang L, Du X, Yang Y (2016) CO2 absorption characteristics in Ammonia solution inside the structured packed column. Ind Eng Chem Res 55(12):3696–3709
Derevschikov VS, Lysikov AI, Okunev AG (2011) High temperature CaO/Y2O3 carbon dioxide absorbent with enhanced stability for sorption-enhanced reforming applications. Ind Eng Chem Res 50(22):12741–12749
Donat F, Florin NH, Anthony EJ, Fennell PS (2012) Influence of high-temperature steam on the reactivity of CaO sorbent for CO2 capture. Environ Sci Technol 46(2):1262–1269
He S, Hu Y, Hu T, Ma A, Jia Q, Su H, Shan S (2017) Investigation of CaO-based sorbents derived from eggshells and red mud for CO2 capture. J Alloys Compd 701:828–833
Hu Y, Jia Q, Shan S, Li S, Jiang L, Wang Y (2015a) Development of CaO-based sorbent doped with mineral rejects–bauxite-tailings in cyclic CO2 capture. J Taiwan Inst Chem Eng 46:155–159
Hu Y, Liu W, Sun J, Li M, Yang X, Zhang Y, Xu M (2015b) Incorporation of CaO into novel Nd2O3 inert solid support for high temperature CO2 capture. Chem Eng J 273:333–343
Koirala R, Reddy GK, Smirniotis PG (2012) Single nozzle flame-made highly durable metal doped ca-based sorbents for CO2 capture at high temperature. Energy Fuel 26(5):3103–3109
Li L, King DL, Nie Z, Howard C (2009a) Magnesia-stabilized calcium oxide absorbents with improved durability for high temperature CO2 capture. Ind Eng Chem Res 48(23):10604–10613
Li Y, Zhao C, Chen H, Liang C, Duan L, Zhou W (2009b) Modified CaO-based sorbent looping cycle for CO2 mitigation. Fuel 88(4):697–704
Li Y, Zhao C, Ren Q, Duan L, Chen H, Chen X (2009c) Effect of rice husk ash addition on CO2 capture behavior of calcium-based sorbent during calcium looping cycle. Fuel Process Technol 90(6):825–834
Liu CT, Li YJ, Sun RY, Xie X (2012) Development of CaO-based sorbent doped with framework materials for CO2 capture. Adv Mater Res 518-523:715–719
Luo C, Zheng Y, Ding N, Zheng C (2011) Enhanced cyclic stability of CO2 adsorption capacity of CaO-based sorbents using La2O3 or Ca12Al14O33 as additives. Korean J Chem Eng 28(4):1042–1046
Ma X, Li Y, Chi C, Zhang W, Shi J, Duan L (2017) CO2 capture performance of mesoporous synthetic sorbent fabricated using carbide slag under realistic calcium looping conditions. Energy Fuel 31(7):7299–7308
Manovic V, Anthony EJ (2009) Screening of binders for pelletization of CaO-based sorbents for CO2 capture. Energy Fuel 23(10):4797–4804
Manovic V, Anthony EJ (2010) Carbonation of CaO-based sorbents enhanced by steam addition. Ind Eng Chem Res 49(19):9105–9110
Martavaltzi CS, Lemonidou AA (2008) Parametric study of the CaO− Ca12Al14O33 synthesis with respect to high CO2 sorption capacity and stability on multicycle operation. Ind Eng Chem Res 47(23):9537–9543
Mason JA, McDonald TM, Bae T-H, Bachman JE, Sumida K, Dutton JJ, Kaye SS, Long JR (2015) Application of a high-throughput analyzer in evaluating solid adsorbents for post-combustion carbon capture via multicomponent adsorption of CO2, N2, and H2O. J Am Chem Soc 137(14):4787–4803
Pacciani R, Müller C, Davidson J, Dennis J, Hayhurst A (2008) Synthetic ca-based solid sorbents suitable for capturing CO2 in a fluidized bed. Can J Chem Eng 86(3):356–366
Peng W, Xu Z, Zhao H (2016) Batch fluidized bed test of SATS-derived CaO/TiO2–Al2O3 sorbent for calcium looping. Fuel 170:226–234
Qin C, Yin J, An H, Liu W, Feng B (2012) Performance of extruded particles from calcium hydroxide and cement for CO2 capture. Energy Fuel 26(1):154–161
Radfarnia HR, Sayari A (2015) A highly efficient CaO-based CO2 sorbent prepared by a citrate-assisted sol–gel technique. Chem Eng J 262:913–920
Ramkumar S, Fan L-S (2010) Calcium looping process (CLP) for enhanced noncatalytic hydrogen production with integrated carbon dioxide capture. Energy Fuel 24(8):4408–4418
Ridha FN, Manovic V, Macchi A, Anthony EJ (2012) High-temperature CO2 capture cycles for CaO-based pellets with kaolin-based binders. Int J Greenhouse Gas Control 6:164–170
Salman M, Cizer Ö, Pontikes Y, Santos RM, Snellings R, Vandewalle L, Blanpain B, Van Balen K (2014) Effect of accelerated carbonation on AOD stainless steel slag for its valorisation as a CO2-sequestering construction material. Chem Eng J 246:39–52
Shan S, Ma A, Hu Y, Jia Q, Wang Y, Peng J (2016) Development of sintering-resistant CaO-based sorbent derived from eggshells and bauxite tailings for cyclic CO2 capture. Environ Pollut 208(Pt B):546–552
Stendardo S, Felice LD, Gallucci K, Foscolo PU (2011) CO2 capture with calcined dolomite: the effect of sorbent particle size. Biomass Conv Bioref 1:149–161
Stendardo S, Andersen LK, Herce C (2013) Self-activation and effect of regeneration conditions in CO2–carbonate looping with CaO–Ca12Al14O33 sorbent. Chem Eng J 220:383–394
Sun R, Li Y, Wu S, Liu C, Liu H, Lu C (2013) Enhancement of CO2 capture capacity by modifying limestone with propionic acid. Powder Technol 233:8–14
Sun J, Liu W, Hu Y, Wu J, Li M, Yang X, Wang W, Xu M (2016) Enhanced performance of extruded–spheronized carbide slag pellets for high temperature CO2 capture. Chem Eng J 285:293–303
Wang K, Guo X, Zhao P, Zheng C (2010) Cyclic CO2 capture of CaO-based sorbent in the presence of metakaolin and aluminum (hydr)oxides. Appl Clay Sci 50(1):41–46
Witoon T, Mungcharoen T, Limtrakul J (2014) Biotemplated synthesis of highly stable calcium-based sorbents for CO2 capture via a precipitation method. Appl Energy 118:32–40
Wu SF, Li QH, Kim JN, Yi KB (2008) Properties of a nano CaO/Al2O3 CO2 sorbent. Ind Eng Chem Res 47(1):180–184
Yan F, Jiang J, Li K, Tian S, Liu Z, Shi J, Chen X, Fei J, Lu Y (2016) Cyclic performance of waste-derived SiO2 stabilized, CaO-based sorbents for fast CO2 capture. ACS Sustain Chem Eng 4(12):7004–7012
Zhang M, Guo Y (2013) Process simulations of large-scale CO2 capture in coal-fired power plants using aqueous ammonia solution. Int J Greenhouse Gas Control 16:61–71
Zhang X, Li Z, Peng Y, Su W, Sun X, Li J (2014) Investigation on a novel CaO–Y2O3 sorbent for efficient CO2 mitigation. Chem Eng J 243:297–304
Zhao M, Bilton M, Brown AP, Cunliffe AM, Dvininov E, Dupont V, Comyn TP, Milne SJ (2014) Durability of CaO–CaZrO3 sorbents for high-temperature CO2 capture prepared by a wet chemical method. Energy Fuel 28(2):1275–1283
Funding
This work has been sponsored by National Natural Science Foundations of China (21766016, 21566014, and 51364023) and the Yunnan Talent Reserve Project (2015HB014).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Zhang, Y., He, L., Ma, A. et al. CaO-based sorbent derived from lime mud and bauxite tailings for cyclic CO2 capture. Environ Sci Pollut Res 25, 28015–28024 (2018). https://doi.org/10.1007/s11356-018-2825-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2825-1