Abstract
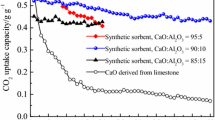
The CO2 capture capacity and cyclic stability of calcium oxide (CaO) prepared from cockle shells (CS) were enhanced by incorporating rice husk (RH) and binder through wet-mixing method. The cyclic reaction of calcination and carbonation was demonstrated using thermal gravimetric analyzer (TGA) which the calcination was performed in a pure N2 environment at 850 °C for 20 min and carbonation at 650 °C for 30 min in 20 vol% of CO2 in N2. The analysis using x-ray fluorescence (XRF) identified silica (Si) as the major elements in the sorbents. The RH-added sorbents also contained several types of metal elements such as which was a key factor to minimize the sintering of the sorbent during the cyclic reaction and contributed to higher CO2 capture capacity. The presence of various morphologies also associated with the improvement of the synthesized sorbents performance. The highest initial CO2 capture capacity was exhibited by CS+10%RH sorbent, which was 12% higher than the RH-free sorbent (CS). However, sorbents with the higher RH loading amount such as 40 and 50 wt% were preferred to maintain high capture capacity when the sorbents were regenerated and extended to the cyclic reaction. The sorbents also demonstrated the lowest average sorption decay, which suggested the most stable sorbent for cyclic-reaction. Once regenerated, the capture capacity of the RH-added sorbent was further increased by 12% when clay was added into the sorbent. Overall, the metal elements in RH and clay were possibly the key factor that enhances the performance of CaO prepared from CS, particularly for cyclic CO2 capture.

Cyclic calcination and carbonation reaction











Similar content being viewed by others
References
Abang S, Janaun J, Anisuzzaman SM, Ikhwan FS (2016) Development of carbon dioxide adsorbent from rice husk char. IOP Conference Series: Earth and Environmental Science 36:12022
Ahiduzzaman M, Sadrul-Islam AKM (2015) Thermo-gravimetric and kinetic analysis of different varieties of rice husk. Procedia Engineering 105(Icte 2014):646–651
Ali LI, El-Molla SA, Amin NH et al (2016) Effect of Ag-doping of nanosized FeAlO system on its structural, surface and catalytic properties. Arab J Chem 9:S1242–S1251
Al-Jeboori MJ, Fennel PS, Nguyen M, Feng K (2012) Effects of different dopants and doping procedures on the reactivity of cao-based sorbents for CO2 capture. Energy Fuels 26(11):6584–6594
Alonso M, Criado YA, Abanades JC, Grasa G (2014) Undesired effects in the determination of CO2 carrying capacities of CaO during TG testing. Fuel 127:52–61
Antzara A, Heracleous E, Lemonidou A (2014) Development of CaO-based mixed oxides as stable sorbents for post-combustion CO2 capture via carbonate looping. Energy Procedia 63:2160–2169
Battegazzore D, Bocchini S, Alongi J, Franche A (2014) Rice husk as bio-source of silica: preparation and characterization of PLA–silica bio-composites. RSC Adv 4(97):54703–54712. https://doi.org/10.1039/c4ra05991c
Benitez-Guerrerro M, Manuel J, Sanchez-Jimenez, Perejon A, Perez-Maqueda L (2018a) Calcium looping performance of mechanically modified Al2O3-CaO composites for energy storage and CO2 capture. Chem Eng J 334:2343–2355
Benitez-Guerrerro M, Valverde JM, Perejon A, Sanchez-Jimenez PE, Perez-Maqueda LA (2018b) Low-cost Ca-based composites synthesized by biotemplate method for thermochemical energy storage of concentrated solar power. Appl Energy 210:108–116
Blamey J, Anthony EJ, Wang J, Fennel PS (2010) The calcium looping cycle for large scale CO2 capture. Prog Energy Combust Sci 36:260–279
Broda M, Kierzkowska AM, Muller CR (2012) Application of the sol-gel technique to develop synthetic calcium-based sorbents with excellent carbon dioxide capture characteristics. ChemSusChem 5(2):411–418
Castilho S, Kiennemann A, Costa-Pereira MF, Soares-Dias AP (2013) Sorbents for CO2 capture from biogenesis calcium wastes. Chem Eng J 226:146–153
Dean CC, Blamey J, Florin NH, Al-Jeboori MJ, Fennel PS (2011) The calcium looping cycle for CO2 capture from power generation, cement manufacturer and hydrogen production. Chem Eng Res Des 89(6):836–855
DOA (2015) Paddy statistics of Malaysia 2014. http://nkea.mardi.gov.my/index.php/epp-pertanian/epp/30-epp9-pengeluaran-beras-wangi-di-kawasan-luar-jelapang-padi
Dou B, Song Y, Liu Y, Feng C (2010) High temperature CO2 capture using calcium oxide sorbent in a fixed-bed reactor. J Hazard Mater 183(1–3):759–765
Dowling A, O’Dwyer J, Adley CC (2015) Lime in the limelight. J Clean Prod 92:13–22
Duan L, Su C, Erans M, Li Y, Anthony EJ, Chen H (2016) CO2 capture performance using biomass-templated cement-supported limestone pellets. Ind Eng Chem Res 55(39):10294–10300
Gupta H, Fan LS (2002) Carbonation−calcination cycle using high reactivity calcium oxide for carbon dioxide separation from flue gas. Ind Eng Chem Res 41(16):4035–4042
Jing JY, Li TY, Zhang XW, Wang SD, Feng J, Turmel WA, Li WY (2017) Enhanced CO2 sorption performance of CaO-Ca3Al2O6 sorbents and its sintering resistance mechanism. Appl Energy 199:225–233
Kawabata CY, Savastano-Junior H, Sousa-Coutinho J (2012) Rice husk derived waste materials as partial cement replacement in lightweight concrete. Ciência e Agrotecnologia 36(5):567–578
Kierzkowska AM, Pacciani R, Muller CR (2013) CaO-based CO2 sorbents: from fundamentals to the development of new, highly effective materials. ChemSusChem 6(7):1130–1148
Kotyczka-Moranska M, Tomaszewicz G, Labojko G (2012) Comparison of different methos for enhcnacing CO2 capture by CaO-based sorbents. Physicochem Probl Mi 48(1):77–90
Kumar S, Saxena SK (2014) A comparative study of CO2 sorption properties for different oxides. Mater Renew Sustain Energy 3(30). https://doi.org/10.1007/s40243-014-0030-9
Laursen K, Grace JR, Lim CJ (2001) Enhancement of the sulfur capture capacity of limestones by the addition of Na2CO3 and NaCl. Environ Sci Technol 35(21):4384–4389
Lee KT, Bhatia S, Mohamed AR (2005) Preparation and characterization of sorbents prepared from ash (waste material) for sulfur dioxide (SO2) removal. J Mater Cycles Waste Manag 7(1):16–23
Li Y, Zhao C, Chen H et al (2009a) CO2 capture behavior of shell during calcination/carbonation cycles. Chem Eng Technol 32(8):1176–1182
Li Y, Zhao C, Ren Q, Duan L, Chen H, Chen X (2009b) Effect of rice husk ash addition on CO2 capture behavior of calcium-based sorbent during calcium looping cycle. Fuel Process Technol 90(6):825–834
Li L, Wen X, Fu X, Wang F, Zhao N, Xiao F, Wei W, Sun Y (2010) MgO/Al2O3 sorbent for CO2 capture. Energy Fuels 24:5773–5780
Liou TZ, Wu SJ (2009) Characteristics of microporous/mesoporous carbons prepared from rice husk under base- and acid-treated conditions. J Hazard Mater 171(1–3):693–703
Liu W, An H, Qin C, Yin J, Wang G, Feng B, Xu M (2012) Performance enhancement of calcium oxide sorbents for cyclic CO2 capture: a review. Energy Fuel 26:2751–2767
Macht F, Eusterhues K, Pronk GJ, Totsche KU (2011) Specific surface area of clay minerals: comparison between atomic force microscopy measurements and bulk-gas (N2) and –liquid (EGME) adsorption methods. Appl Clay Sci 53:20–26
Mahinpey N, Sedghkerdar MH, Aqsha A, Soleimanisalim AH (2016) CO2 capture performance of core/shell CaO-based sorbent using mesostructured silica and titania in a multicycle CO2 capture process. Ind Eng Chem Res 55(16):4532–4538
Manovic V, Anthony EJ (2010) Sintering and formation of a nonporous carbonate shell at the surface of CaO-based sorbent particles during CO2-capture cycles. Energy Fuels 24(10):5790–5796
Manovic V, Charland JP, Blamey J, Fennell PS, Lu DY, Anthony EJ (2009) Influence of calcination conditions on carrying capacity of CaO-based sorbent in CO2 looping cycles. Fuel 88:1893–1900
Martinez I, Grasa G, Murillo R, Arias B, Abanades JC (2012) Kinetics of calcination of partially carbonated particles in a Ca-looping system for CO2 capture. Energy Fuels 26(2):1432–1440
Martos A et al (2016) Effect of dolomite decomposition under CO2 on its multicycle CO2 capture behaviour under calcium looping conditions. Phys Chem Chem Phys 18:16325–16336
Mohamed RM, Mkhalid IA, Barakat MA (2015) Rice husk ash as a renewable source for the production of zeolite NaY and its characterization. Arab J Chem 8(1):48–53
Mohammadi M, Lahijani P, Mohamed AR (2014a) Refractory dopant-incorporated CaO from waste eggshell as sustainable sorbent for CO2 capture: experimental and kinetic studies. Chem Eng J 243:455–464
Mohammadi M, Lahijani P, Zainal ZA, Mohamed AR (2014b) Capture of carbon dioxide from flue/fuel gas using dolomite under microwave irradiation. Chem Eng J 240:169–178
Ni M, Ratner BD (2008) Differentiation of calcium carbonate polymorphs by surface analysis technique—an XPS and TOF-SIMS study. Surf Interface Anal 40(10):1356–1361
Olajire AA (2010) CO2 capture and separation technologies for end-of-pipe applications- A review. Energy 35:2610–2628
Olszak-Humienik M, Jablonski M (2015) Thermal behavior of natural dolomite. J Them Anal Calorim 119(3):2239–2248
Phromprasit J, Powell J, Assabumrungrat S (2016) Metals (Mg, Sr, and Al) modified CaO based sorbent for CO2 sorption/desorption stability in fixed bed reactor for high temperature application. Chem Eng J 284:1212–1223
Reddy EP, Smirniotis PG (2004) High-temperature sorbents for CO2 made of alkali metals doped on CaO supports. J Phys Chem B 108(23):7794–7800
Salaudeen SA, Acharya B, Dutta A (2018) CaO-based CO2 sorbents: a review on screening, enhancement, cyclic stability, regeneration and kinetics modelling. J CO2 Util 23:179–199
Samanta A, Zhou A, Shimizu GKH et al (2012) Post-combustion CO2 capture using solid sorbents: a review. Ind Eng Chem Res 51(4):1438–1463
Shi J, Li Y, Zhang Q, Ma X, Duan L, Zhou X (2017) CO2 capture performance of a novel synthetic CaO/sepiolite sorbent at calcium looping conditions. Appl Energy 203:412–421
Singh M, Vinodkumar S, Waghmare SA, Sabale PD (2016) Aragonite-vaterite-calcite: polymorphs of CaCO3 in 7th century CE lime plasters of Alampur group temples India. Constr Build Mater 112:386–397
Stanmore BR, Gilot P (2005) Review-calcination and carbonation of limestone during thermal cycling for CO2 sequestration. Fuel Process Technol 86(16):1707–1743
Valverde JM (2013) Ca-based synthetic materials with enhanced CO2 capture efficiency. J Mater Chem A 1(3):447–468
Valverde JM, Sanchez-Jimenez PE, Perez-Maqueda LA, Quitanilla MAS, Perez-Vaquero J (2014) Role of crystal structure on CO2 capture by limestone derived CaO subjected to carbonation/recarbonation/calcination cycles at ca-looping conditions. Appl Energy 125:264–275
Vu AT, Park Y, Jeon PR, Lee CH (2014) Mesoporous MgO sorbent promoted with KNO3 for CO2 capture at intermediate temperatures. Chem Eng J 258:254–264
Witoon T (2011) Characterization of calcium oxide derived from waste eggshell and its application as CO2 sorbent. Ceram Int 37:3291–3298
Wu SF, Beum TH, Yang JI, Kim JN (2007) Properties of Ca-base CO2 sorbent using Ca(OH)2 as precursor. Ind Eng Chem Res 46:7896–7899
Yu FC, Phalak N, Sun Z, Fan LS (2012) Activation strategies for calcium-based sorbents for CO2 capture: a perspective. Ind Eng Chem Res 51(4):2133–2142
Zakaria Z, Mohd-Ishak MA, Abdullah MF, Ismail K (2010) Thermal decomposition study of coals, rice husk, rice husk char and their blends during pyrolysis and combustion via thermogravimetric analysis. Int J Chem Technol 2(3):78–87
Zhang L, Lu Y, Rostam-Abadi M (2012) Sintering of calcium oxide (CaO) during CO2 chemisorption: a reactive molecular dynamics study. Phys Chem Chem Phys 14(48):16633–16643
Acknowledgments
The authors would like to acknowledge Universiti Teknologi PETRONAS and Kida Laboratory, Kumamoto University, Japan, for providing the research facilities. The authors are also grateful to Bernas Sg. Renggam in Perak, Malaysia, for their generosity in providing rice husk materials.
Funding
This study received financial support from the Universiti Teknologi PETRONAS (the Biomass Research Grant (0153AB-F02) and Kida Laboratory, Kumamoto University, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Mohamed, M., Yusup, S., Quitain, A.T. et al. Utilization of rice husk to enhance calcium oxide-based sorbent prepared from waste cockle shells for cyclic CO2 capture in high-temperature condition. Environ Sci Pollut Res 26, 33882–33896 (2019). https://doi.org/10.1007/s11356-018-2549-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2549-2