Abstract
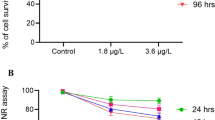
As a universal environmental contaminant, the herbicide cyhalofop-butyl is considered to have infested effects on the embryonic development of aquatic species. The present study focused on an assessment of the impacts of cyhalofop-butyl on Yellow River carp embryos. It was found that cyhalofop-butyl inhibited the hatching of the embryos, and the hatching rate decreased with higher concentrations of the herbicide. The mortality rate was increased on exposure to cyhalofop-butyl and was significantly higher in the 1.6 and 2 mg/L treatment groups over 48 h. All of the embryos of the 2 mg/L treatment group died within the 48 h post-hatching stage. And the transcription of several embryos related to apoptosis was also influenced by cyhalofop-butyl exposure. Further, cyhalofop-butyl exposure leads to a series of morphological changes (pericardial edema, tail deformation, and spine deformation) in embryos, which were consistent with significant modifications in the associated genes. These results provided a scientific basis for further studies into the effects of cyhalofop-butyl on aquatic organisms.








Similar content being viewed by others
References
Abrahams A, Parker MI, Prince S (2010) The T-box transcription factor Tbx2: its role in development and possible implication in cancer. IUBMB Life 62(2):92–102. https://doi.org/10.1002/iub.275
Balemans W, Van Hul W (2002) Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol 250(2):231–250
Black BL, Olson EN (1998) Transcriptional control of muscle development by myocyte enhancer factor 2 (MEF2) proteins. Annu Rev Cell Dev Biol 14:167–196
Bone Q (1978) Locomotor muscle. Fish Physiology:361–424
Canalis E, Economides AN, Gazzerro E (2003) Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev 24(2):218–235
Cao F, Liu X, Wang C, Zheng M, Li X, Qiu L (2016) Acute and short-term developmental toxicity of cyhalofop-butyl to zebrafish (Danio rerio). Environ Sci Pollut Res Int 23(10):10080–10089. https://doi.org/10.1007/s11356-016-6236-x
Cao JJ, Li M, Li SG (2011) Development strategy research of modern eco-agriculture on the basis of constructing the rural circular economy-for the example of Shandong province. Energy Procedia:2504–2250
Chong M (2007) Expression and function of tbx2 gene related to heart development in zebrafish. FuDan University R725:4
de Pazini B, J, Pasini RA, Rakes M, de Armas FS, Seidel EJ, da S Martins JF, Grützmacher AD (2017) Toxicity of pesticide tank mixtures from rice crops against Telenomus podisi Ashmead (Hymenoptera: Platygastridae). Neotrop Entomol 46(4):461–470. https://doi.org/10.1007/s13744-017-0483-5
De la Paz JF, Beiza N, Paredes-Zúñiga S, Hoare MS, Allende ML (2017) Triazole fungicides inhibit zebrafish hatching by blocking the secretory function of hatching gland cells. Int J Mol Sci 18. https://doi.org/10.3390/ijms18040710
Ducharme NA, Reif DM, Gustafsson JA, Bondesson M (2015) Comparison of toxicity values across zebrafish early life stages and mammalian studies: implications for chemical testing. Reprod Toxicol 55:3–10. https://doi.org/10.1016/j.reprotox.2014.09.005
Graff JM (1997) Embryonic patterning: to BMP or not to BMP, that is the question. Cell 89(2):171–174
Hinits Y, Hughes SM (2007) Mef2s are required for thick filament formation in nascent muscle fibres. Development 134(13):2511–2519
Hinits Y, Pan L, Walker C, Dowd J, Moens CB, Hughes SM (2012) Zebrafish Mef2ca and Mef2cb are essential for both first and second heart field cardiomyocyte differentiation. Dev Biol https://doi.org/10.1016/j.ydbio.2012.06.019
Hogan BL (1996) Bone morphogenetic proteins in development. Curr Opin Genet 6:432–438
Huang F (2007) Studies on residue analysis of baicchu in paddyfield and its toxicity to two living things.[D] Hunan Agricultural University X173
Huang F, Guo Z, Xu Z, Yang R (2007) Toxicity of cyhalofop-butyl and fenoxaprop-ethyl to tadpole. Agro-Environ Sci 1063e1066
Jiang Y, Zhang S, Xu J, Feng J, Mahboob S, Al-Ghanim KA, Sun X, Xu P (2014) Comparative transcriptome analysis reveals the genetic basis of skin color variation in common carp. PLoS One 9(9):e108200. https://doi.org/10.1371/journal.pone.0108200
Jin M, Zhang X, Wang L, Huang C, Zhang Y, Zhao M (2009) Developmental toxicity of bifenthrin in embryo-larval stages of zebrafish. Aquat Toxicol 95(4):347–354. https://doi.org/10.1016/j.aquatox.2009.10.003
Jin Y, Liu Z, Peng T, Fu Z (2015) The toxicity of chlorpyrifos on the early life stage of zebrafish: a survey on the endpoints at development, locomotor behavior, oxidative stress and immunotoxicity. Fish Shellfish Immunol 43(2):405–414. https://doi.org/10.1016/j.fsi.2015.01.010
Kablar B, Krastel K, Ying C, Asakura A, Tapscott SJ, Rudnicki MA (1997) MyoD and Myf-5 differentially regulate the development of limb versus trunk skeletal muscle. Development 124(23):4729–4738
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4):239–257
Khan ZR, James DG, Midega CAO, Pickett JA (2008) Chemical ecology and conservation biological control. Biol Control 45(1):210–224
Kumar B, Sharma R, Singh SB (2012) Evaluation of harvest residues of cyhalofop-butyl in paddy soil. Bull Environ Contam 89(2):344–347. https://doi.org/10.1007/s00128-012-0701-0
Li K, Wu JQ, Jiang LL, Shen LZ, Li JY, He ZH, Wei P, Lv Z, He MF (2017) Developmental toxicity of 2,4-dichlorophenoxyacetic acid in zebrafish embryos. Chemosphere 171:40–48. https://doi.org/10.1016/j.chemosphere.2016.12.032
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25(4):402–408
Li Y, Hallerman EM, Liu Q, Wu K, Peng Y (2016) The development and status of Bt rice in China. Plant Biotechnol J 14(3):839–848. https://doi.org/10.1111/pbi.12464
Lou Y (1965) The hatching enzymes of fish. Chinese Journal of Zoology. doi:https://doi.org/10.13859/j.cjz.1965.03.003
Mackey TJ, Borkowski A, Amin P, C Jacobs S C, Kyprianou N (1998) Bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with prostate cancer. Urology 52 (6) (1998) 1085–1090
Manjunatha B, Peng W, Liu K, Marigoudar SR, Chen X, Wang X, Wang X (2014) The effects of henna (hair dye) on the embryonic development of zebrafish (Danio rerio). Environ Sci Pollut Res Int 21(17):10361–10367. https://doi.org/10.1007/s11356-014-2968-7
Mariani FV, Martin GR (2003) Deciphering skeletal patterning: clues from the limb. Nature 423(6937):319–325
Meilhac SM, Esner M, Kerszberg M (2004) Oriented clonal cell growth in the developing mouse myocardium underlies cardiac morphogenesis. J Cell Biol 164(1):97–109
Molkentin JD, Black BL, Martin JF, Olson EN (1995) Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 83(7):1125–1136
Moraco AH, Kornfeld H (2014) Cell death and autophagy in tuberculosis. Semin Immunol 26(6):497–511. https://doi.org/10.1016/j.smim.2014.10.001
Mu X, Chai T, Wang K, Zhu L, Huang Y, Shen G, Li Y, Li X, Wang C (2016) The developmental effect of difenoconazole on zebrafish embryos: a mechanism research. Environ Pollut 212:18–26. https://doi.org/10.1016/j.envpol.2016.01.035
Niell S, Pareja L, Geis Asteggiante L, Cesio MV, Heinzen H (2010) Comparison of extraction solvents and conditions for herbicide residues in milled rice with liquid chromatography-diode array detection analysis (LC-DAD). Food Addit Contam Part A Chem Anal Control Expo Risk Assess 27(2):206–211. https://doi.org/10.1080/19440040903296246
Ola MS, Nawaz M, Ahsan H (2011) Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem 351(1–2):41–58. https://doi.org/10.1007/s11010-010-0709-x
Pileczki V, Cojocneanu-Petric R, Maralani M, Neagoe IB, Sandulescu R (2016) MicroRNAs as regulators of apoptosis mechanisms in cancer. Clujul Med 89(1):50–55. https://doi.org/10.15386/cjmed-512
Potthoff MJ, Arnold MA, McAnally J, Richardson JA, Bassel-Duby R, Olson EN (2007) Regulation of skeletal muscle sarcomere integrity and postnatal muscle function by Mef2c. Mol Cell Biol 27(23):8143–8151
Qin L, Liu F, Liu H, Wei Z, Sun P, Wang Z (2014) Evaluation of HODE-15, FDE-15, CDE-15, and BDE-15 toxicity on adult and embryonic zebrafish (Danio rerio). Environ Sci Pollut Res Int 21(24):14047–14057. https://doi.org/10.1007/s11356-014-3322-9
Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i AA, Tanabe Y, Placzek M, Edlund T, Jessell TM (1994) Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell 76(4):761–775
Schindler YL, Garske KM, Wang J, Firulli BA, Firulli AB, Poss KD, Yelon D (2014) Hand2 elevates cardiomyocyte production during zebrafish heart development and regeneration. Development 141(16):3112–3122. https://doi.org/10.1242/dev.106336
Smith A, Avaron F, Guay D, Padhi BK, Akimenko MA (2006) Inhibition of BMP signaling during zebrafish fin regeneration disrupts fin growth and scleroblasts differentiation and function. Dev Biol 299(2):438–454
Someya S, Tanokura M, Salvi R (2011) Detection of apoptosis by RT-PCR array in mefloquine-induced cochlear damage. J Otology. https://doi.org/10.13489/j.cnki.11-4883/r.2011.01.003
Stennard FA, Harvey RP (2005) T-box transcription factors and their roles in regulatory hierarchies in the developing heart. Development 132(22):4897–4910
Sun M, Wang M, Yu L, Zhu Y, Dong X, Xiao Q, Liu Y, Sun H, Duan J, Gao T (2017) Residue and decline study of cyhalofop-butyl and bispyribac-sodium in paddy water and soil by matrix solid-phase dispersion and liquid chromatography-mass spectrometry. Environ Chem 36(3):691–694
Thies RS, Bauduy M, Ashton BA, Kurtzberg L, Wozney JM, Rosen V (1992) Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology 130(3):1318–1324
Togi K, Kawamoto T, Yamauchi R (2004) Role of Hand1/eHAND in the dorso-ventral patterning and interventricular septum formation in the embryonic heart. Mol Cell Biol 24(11):4627–4635
Ulukaya E, Acilan C, Yilmaz Y (2011) Apoptosis: why and how does it occur in biology? Cell Biochem Funct 29(6):468–480. https://doi.org/10.1002/cbf.1774
Verzi MP, Agarwal P, Brown C, McCulley DJ, Schwarz JJ, Black BL (2007) The transcription factor MEF2C is required for craniofacial development. Dev Cell 12:645–652
Vincentz JW, Toolan KP, Zhang W, Firulli AB (2017) Hand factor ablation causes defective left ventricular chamber development and compromised adult cardiac function. PLoS Genet 13(7):e1006922. https://doi.org/10.1371/journal.pgen.1006922
Wang SF, Liu KC, Wang XM, He QX, Chen XQ (2011) Toxic effects of celastrol on embryonic development of zebrafish (Danio rerio). Drug Chem Toxicol 34(1):61–65. https://doi.org/10.3109/01480545.2010.494664
Wan M, Cao X (2005) BMP signaling in skeletal development. Biochem Biophys Res Commun 328(3):651–657
Wu C, Zhao X, Wu S (2011) Toxicity and risk of cyhalofop-butyl to Rana limnocharis. Acta Agriculturae Zhejiangensis 1004–1524 (2011) 04–0771-05
Wu MH, Jin XK, Yu AQ, Zhu YT, Li D, Li WW (2014) Caspase-mediated apoptosis in crustaceans: cloning and functional characterization of EsCaspase-3-like protein from Eriocheir. Fish Shellfish Immunol 41(2):625–632
Xia X, Xia X, Huo W, Dong H, Zhang L, Chang Z (2016) Toxic effects of imidacloprid on adult loach (Misgurnus anguillicaudatus). Environ Toxicol Pharmacol 45:132–139. https://doi.org/10.1016/j.etap.2016.05.030
Xia X, Zhang L, Si S (2013) Acute toxicity and physiological toxicity of lambda-cyhalothrin to Misgurnus anguillicaudatus. Jiangsu Agric Sci 41(6):270–272
Yamaguchi A, Katagiri T, Ikeda T, Wozney JM, Rosen V, Wang EA, Kahn AJ, Suda T, Yoshiki S (1991) Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J Cell Biol 113(3):681–687
Yoon BS, Lyons KM (2004) Multiple functions of BMPs in chondrogenesis. J Cell Biochem 93(1):93–103
Yue D, Zhang X, Zhao F, Ru S (2016) Study of the toxicity of monocrotophos on the cardiac and skeletal development of Danio rerio embryo. Periodical of Ocean University of China. DOI:https://doi.org/10.16441/j.cnki.hdxb.20150410
Yukio K, Kawamoto T, Fujimoto K, Noshiro M (2014) DEC1/STRA13/SHARP2 and DEC2/SHARP1 coordinate physiological processes, including circadian rhythms in response to environmental stimuli. Curr Top Dev Biol 110:339–372. https://doi.org/10.1016/B978-0-12-405943-6.00010-5
Yu K, Li G, Feng W, Liu L, Zhang J, Wu W, Xu L, Yan Y (2015) Chlorpyrifos is estrogenic and alters embryonic hatching, cell proliferation and apoptosis in zebrafish. Chem Biol Interact 239:26–33. https://doi.org/10.1016/j.cbi.2015.06.010
Zeng C, Sun H, Xie P, Wang J, Zhang G, Chen N, Yan W, Li G (2014) The role of apoptosis in MCLR-induced developmental toxicity in zebrafish embryos. Aquat Toxicol 149:25–32. https://doi.org/10.1016/j.aquatox.2014.01.021
Zhu L, Mu X, Wang K, Chai T, Yang Y, Qiu L, Wang C (2015) Cyhalofop-butyl has the potential to induce developmental toxicity, oxidative stress and apoptosis in early life stage of zebrafish (Danio rerio). Environ Pollut 203:40–49. https://doi.org/10.1016/j.envpol.2015.03.044
Funding
This work is supported by grants from the National Natural Science Foundation of China (no. 31200923), Young Core Instructor Foundation of Henan Normal University (no. 5101049470610), and Program for Innovative Research Team (in Science and Technology) in University of Henan Province (no. 17IRTSTHN017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Supplementary Fig. 1
(JPG 27 kb)
Rights and permissions
About this article
Cite this article
Xia, X., Wang, P., Wan, R. et al. Toxic effects of cyhalofop-butyl on embryos of the Yellow River carp (Cyprinus carpio var.): alters embryos hatching, development failure, mortality of embryos, and apoptosis. Environ Sci Pollut Res 25, 24305–24315 (2018). https://doi.org/10.1007/s11356-018-2489-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2489-x