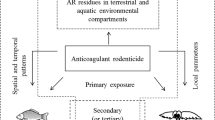
Abstract
Anticoagulant rodenticides (ARs) have been used for decades for rodent control worldwide. Research on the exposure of the environment and accumulation of these active substances in biota has been focused on terrestrial food webs, but few data are available on the impact of ARs on aquatic systems and water organisms. To fill this gap, we analyzed liver samples of bream (Abramis brama) and co-located suspended particulate matter (SPM) from the German Environmental Specimen Bank (ESB). An appropriate method was developed for the determination of eight different ARs, including first- and second-generation ARs, in fish liver and SPM. Applying this method to bream liver samples from 17 and 18 sampling locations of the years 2011 and 2015, respectively, five ARs were found at levels above limits of quantifications (LOQs, 0.2 to 2 μg kg−1). For 2015, brodifacoum was detected in 88% of the samples with a maximum concentration of 12.5 μg kg−1. Moreover, difenacoum, bromadiolone, difethialone, and flocoumafen were detected in some samples above LOQ. In contrast, no first generation AR was detected in the ESB samples. In SPM, only bromadiolone could be detected in 56% of the samples at levels up to 9.24 μg kg−1. A temporal trend analysis of bream liver from two sampling locations over a period of up to 23 years revealed a significant trend for brodifacoum at one of the sampling locations.
Similar content being viewed by others
Introduction
Since the introduction of warfarin as the first anticoagulant rodenticide on the US market in the late 1940s, rodent control worldwide has relied increasingly upon the use of these chemicals. As of 2017, anticoagulant rodenticides constitute more than 95% of the authorized rodenticides as biocides in the European Union (ECHA 2017b). The discovery of anticoagulant rodenticides (ARs) is today recognized as the most important step towards safer and more effective rodent control (Buckle and Eason 2015).
ARs comprise active substances belonging either to the class of 4-hydroxycoumarines such as warfarin or to 1,3-indandione derivatives such as chlorophacinone. Without regard to their chemical structure, ARs are grouped by their ability to prevent blood clotting (coagulation) by the inhibition of vitamin K which is essential for the production of several blood clotting factors such as prothrombin. Typical symptoms of AR intoxication, i.e., internal and external hemorrhages due to the increased permeability of blood vessels, occur several days after consumption of the rodenticide bait. This delayed mode of action is key to the effectiveness of ARs as it overcomes the bait shyness of rats.
Anticoagulant rodenticides are usually divided into first- and second-generation anticoagulant rodenticides (FGARs/SGARs) depending on the date of their introduction on the market. FGARs (i.e., warfarin, chlorophacinon, coumatetralyl) were firstly used in the late 1940s, 1950s, and 1960s, while SGARs (i.e., bromadiolone, difenacoum, brodifacoum, difethialone, flocoumafen) were developed in the 1970s and 1980s following an increasing concern about warfarin-resistant rodents. Ever since, ARs have been extensively used as pesticides to reduce human and animal infections by rodent-borne diseases, for crop protection against voles, or for species conservation on oceanic islands (Masuda et al. 2015). They are nowadays regulated in the European Union (EU) under the Biocidal Products Regulation (EU) No. 528/2012 (BPR) and the Plant Protection Products Regulation (EC) No. 1107/2009 (PPPR), depending on their intended use to either protect human health, animal health and materials or plants and plant products. Both regulations foresee that ARs need to be authorized prior to being made available on the European market. Under the PPPR, difenacoum (Reg. (EU) No. 540/2011) and bromadiolone (Reg. (EU) No. 540/2011) are the only anticoagulant active substances which are approved for the use in plant protection products in the EU. Under the BPR, the approval of eight anticoagulants, i.e., warfarin, chlorophacinone, coumatetralyl, bromadiolone, difenacoum, brodifacoum, difethialone, and flocoumafen as active substances for the use in rodenticides, have just recently been renewed. While the last authorization of an anticoagulant rodenticide as a plant protection product in Germany has expired in 2015, their authorizations as biocides in Germany have recently been prolonged (BVL 2017). As of September 2017, 704 rodenticide products were authorized in Germany under the BPR, of which about 91% contained an anticoagulant active substance, of these 12.2% FGAR and 79.0% SGAR (compare Table 1) (BAuA 2017).
The environmental risk assessment of ARs under the BPR authorization in the EU revealed high risks of primary and secondary poisoning for non-target organisms, which either feed directly on the bait or consume poisoned rodents. Moreover, all SGARs have been identified as being either persistent, bioaccumulative, and toxic (PBT-substances) or very persistent and very toxic (vPvB-substances). These inherent substance properties in combination with the given exposure of non-target organisms via primary and secondary poisoning and the extensive and widespread use of ARs are significant drivers for the likewise widespread contamination of various wildlife species worldwide. It is thus not surprising that residues of anticoagulant rodenticides, especially of the second-generation compounds, have been detected in a large variety of species. Residues of rodenticides were detected for example, in barn owls (Geduhn et al. 2016, Newton et al. 1990), tawny owls (Walker et al. 2008), common buzzards (Berny et al. 1997), golden eagles (Langford et al. 2013), polecats/mink (Elmeros et al. 2018, Fournier-Chambrillon et al. 2004, Ruiz-Suarez et al. 2014, 2016, Shore et al. 2003), weasels (McDonald et al. 1998), stoats (Elmeros et al. 2011), foxes (Berny et al. 1997, Geduhn et al. 2015, McMillin et al. 2008, Tosh et al. 2011), hedgehogs (Dowding et al. 2010), and snails (Alomar et al. 2018).
Most of these environmental monitoring studies focused on the terrestrial compartment, e.g., predatory birds (Gomez-Ramirez et al. 2014, Ruiz-Suarez et al. 2014, Stansley et al. 2014, Thomas et al. 2011) and mammals (Quinn et al. 2012), as well as various non-target rodents (Elliott et al. 2014, Geduhn et al. 2014). However, little to nothing is known so far, about the exposure of aquatic life to ARs and the accumulation of ARs in aquatic food webs.
The environmental exposure assessment within the authorization of anticoagulant rodenticides under the BPR is based on the Emission Scenario Document (ESD) (Larsen 2003) which considers four main scenarios for the application of ARs, i.e., the application in and around buildings, in open areas (in rate holes), at waste dumps, and in the sewer system. Significant releases to surface water bodies are only assumed to occur from the application of ARs in the latter area of use, i.e., in sewer systems. It has been shown that AR can enter sewage treatment plants (STPs) and thereafter contribute to the loads of anticoagulants to receiving surface waters with effluents (Gomez-Canela et al. 2014). A maximum release to the sewerage system and consequently to surface water could result directly from the application of rodent bait into manholes of the sewer system and indirectly from the target animals’ urine, feces, and dead bodies. The application of rodenticides in rainwater sewers which as a rule are not connected to a sewage treatment plant and discharge directly into receiving waters can be considered another release pathway.
Environmental monitoring of AR provides some specific challenges to the investigator. AR can enter the environment via different exposure routes where they have been shown to exhibit acute toxic effects at concentrations in the ppm and ppb range (e.g., bromadiolone (Eason et al. 2002, Thomas et al. 2011): LC50 of 2.86 mg L−1 for fish, Lepomis macrochirus; LD50 of 0.56 mg kg−1 in rat (oral) (ECHA 2010), or difethialone (ECHA 2007): EC50 of 4.4 μg L−1 for Daphnia magna acute, or LC50 of 51 μg L−1 for Oncorhynchus mykiss. SGARs in particular exhibit a high lipophilicity and environmental persistence and may thus enrich in predator tissues with high fat contents, e.g., mammalian liver (Eason et al. 2002, Thomas et al. 2011), which are complex matrices and thus require elaborate and challenging sample preparation. Furthermore, there are numerous AR substances that may enter the environment and so a comprehensive assessment of the presence of AR requires very sensitive and accurate multi-methods, covering a wide range of different ARs. Several analytical approaches for multi-methods for the quantitative determination of AR in biological samples have been developed, such as liquid chromatography (LC) and also ion chromatography (IC) coupled to tandem mass spectrometry (MS/MS) (Bidny et al. 2015, Chen et al. 2009, Jin et al. 2009, Jin et al. 2008, Marek and Koskinen 2007), two-dimensional LC coupled to MS/MS (Marsalek et al. 2015), IC coupled to fluorescence detection (Jin et al. 2007), methods using high resolution MS (Schaff and Montgomery 2013), and some other strategies that are presented in a review by Imran et al. (2015). Available analytical methods are so far hampered by the number of captured AR, as well as high limits of detection caused by complex biological and environmental matrices that are in contrast to low relevant environmental concentrations. However, the method we apply here is in good agreement (Hernandez et al. 2013) or better (Vandenbroucke et al. 2008a, Vandenbroucke et al. 2008b, Zhu et al. 2013) in terms of number of analytes covered and sensitivity with other LC-MS/MS-based methods for solid biological tissues such as liver and hair.
Good insight is available on risks of AR towards non-target mammals as well as exposure and associated risks of various predators (Christensen et al. 2012, Geduhn et al. 2016, Gomez-Ramirez et al. 2014, Hughes et al. 2013, Langford et al. 2013, Nogeire et al. 2015, Proulx and MacKenzie 2012, Rattner et al. 2014, 2015, Ruiz-Suarez et al. 2014, Thomas et al. 2011).
Even if concentrations are assumed to be low after systematic or accidental exposure of aquatic systems (Fisher et al. 2012, Primus et al. 2005), the environmental impact may yet be relevant due to the high bioaccumulation potential, especially of SGARs (Masuda et al. 2015). So far, no studies are available on AR residues and accumulation in fish or distribution of AR in natural aquatic systems. The aim of this study was to assess the exposure of freshwater fish to anticoagulant rodenticides by analyzing levels of anticoagulants in fish tissues. For this purpose, a highly sensitive and specific multi-method was developed to determine eight anticoagulants, which have been approved under the BPR for the use in rodenticides within the EU (cf. Table 2). We the applied this method in a spatial monitoring study for two time points for fish liver and one for suspended particulate matter (SPM) samples of the German Environmental Specimen Bank (ESB). Finally, retrospective analysis was performed for SPM and fish samples from selected sites to detect time trends. In addition, selected liver samples from otters (Lutra lutra) were analyzed to characterize the bioaccumulation potential of ARs in fish-eating mammal species.
Materials and methods
Collection and storage of samples
All samples were retrieved from the archive of the German Environmental Specimen Bank.
Bream (Abramis brama) samples were analyzed from 17 and 18 sampling locations for 2011 and 2015, respectively, and from 10 sampling years for two specific sampling locations. Sampling locations included 16 riverine sites and one (2011) and two (2015) lakes. Samples were processed and stored according to a dedicated ESB standard operating procedures (SOP) by Klein et al. (2012). SPM was analyzed from the 16 riverine sampling sites sampled in 2015. SPM was collected and processed according to a specific SOP by Ricking et al. (2012).
Otter samples originate from the Upper Lusatia area in the east of Germany (partly Elbe catchment) and represent individuals that died as a result of traffic accidents or lethal diseases. The liver samples of five otter individuals were prepared and analyzed according to the protocol for bream liver samples.
Method summary
In order to optimize and merge available methods, and to secure the specificity of the method, the rodenticide analysis was performed on a UHPLC-chromatographic unit coupled to a high-resolution mass spectrometer operating at a resolution of 35,000. The adopted method mainly based on Thomas et al. (2011) for fish liver could be used to determine a total of eight different target molecules, as given in Table 2 and Table 3. The specificity of the method is assured by measuring the accurate mass of the analytes in tandem mass spectrometric mode (MS/MS) (see Table 3).
Table 2 harbors information on the reference substances used for quantification. Stable isotopically labeled (deuterated) internal standards (IS) were only available for bromadiolone (as D5; Campro Scientific, Germany, 99% D, 95% chemical, Lot # AB126P2), warfarin (as D5; Campro Scientific, Germany, 99% D, 99% chemical, Lot # E305P28), and chlorophacinone (as D4; Chiron AS, Norway, 99.4% D, 99% chemical Lot # 14266). The IS were added to the samples, but not used for evaluation in the final method.
A sample of about 0.5 g fish matrix (liver or muscle; frozen, cryo-milled ESB material) is mixed with roughly 3.5 g Na2SO4 (ratio 1:7), 100 μL IS solution (three IS, each 100 ng mL−1), and 5 mL acetone in a 15-mL polypropylene test tube. This mixture is treated for 30 min in an ultra-sonic bath and for the same time on a vortex shaker. Subsequently, the test tube is centrifuged at 4000 rpm for 5 min. The clear supernatant is forwarded to a fresh test tube, whereas the pellet is extracted with an additional 4 mL volume of fresh acetone. The combined extracts were mixed with 1 mL of diethyl ether and evaporated in a N2-stream at 50 °C to dryness. The remaining extract was then dissolved in 1 mL methanol and homogeneously mixed by treating for 5 min in an ultra-sonic bath. The slightly turbid suspension was forwarded to a 1.5-mL tube and centrifuged at 15,000 rpm for 2 min. The cleared supernatant was finally filtrated through a 25-mm diameter, 0.45-μm regenerated cellulose (RC) type membrane filter, before filling into a UHPLC (ultra-high performance liquid chromatography) vial for analysis.
A minimum of two solvent-based blank samples (up to four) were analyzed in every measurement series. Matrix-based quality control samples containing the rodenticides in defined concentrations (adapted to the expected concentration range in the samples: here 1.4 and 14 μg kg−1 for otter and SPM, and 1.0 and 10 μg kg−1 for bream liver) were measured about every 15 samples. Suitable rodenticide free matrices were identified in a preliminary screening. All samples were measured at least in duplicate, as specified in the respective table captions.
Instrumental parameters
A UHPLC Acquity (Waters), coupled to an Orbitrap Q-Exactive Plus (Thermo Scientific) high-resolution mass spectrometer, run in the multiple reaction monitoring mode with electrospray negative (ES-) ionization was used for all chemical analyses. Accurate masses of parent and daughter ions, as well as MS-parameters, were according to Table 3. The used column was 100 × 2 mm BEH C18, 1.7 μm (Waters), the column temperature was 55 °C, and 20 μL sample volume was injected and run with a flow of 0.35 mL min−1. The solvents used were A: methanol +2 mM ammonium acetate in water (5 + 95, v/v) and B: methanol containing 2 mM ammonium acetate. The used UHPLC gradient program was 0 min 100% A → 10 min 100% B → 13 min 100% B → 15 min 100% A. Under the given conditions, of bromadiolone, two diastereomeric partners elute, which are reported here as a sum.
Method development and method validation
Initially, a comparison of AR concentrations in bream liver and fillet was performed by applying a crude preliminary method that had not been optimized. For difethialone and brodifacoum, we found 100- or 80-fold higher concentrations in liver, respectively. So, it was decided to focus on bream liver samples for further method development and subsequent analysis of environmental samples.
Commercial stable isotope labeled standards were purchased to improve the method. After repeated measurement cycles and calibrations, however, it was found that the analytical parameters are much better when using an external matrix matched calibration. So, the final method does not use the signals for the IS, but an external matrix calibration.
Calibration and validation of the method were performed by standard addition techniques using matrix calibrations in the range from 0.02 to 20.0 μg kg−1 and were evaluated to the lowest calibration level within the linear range of a calibration. Each calibration solution contained 100 μL of IS solution which were spiked with 25 μg L−1, resulting in 5 μg kg−1 of each IS. The handling and measurement of the calibration and validation samples were identical to the treatment of the test samples. For validation of the method, six bream liver and SPM samples of 0.5 g each were fortified with defined AR at individual limit of quantification (LOQ) concentrations to prove for accuracy, repeatability, and precision at the LOQ level (standard addition technique), according to Table 4. Each sample was fortified with 100 μL of a solution containing all rodenticides in the respective concentrations ranging from 0.1 to 100 μg L−1 and with 100 μL of the IS solution. Otter liver was used to generate a respective matrix calibration, but due to limited sample material, no separate otter liver validation could be performed.
Due to varying sensitivities of individual AR, the dynamic ranges of the calibrations are different, but none of them showed an exponential behavior. To keep the procedure constant, even after the decision to omit using the IS, their addition to the samples was continued. For the given calibration ranges, all functions were linear and show coefficients of determination (r2) of at least 0.99. The validated limits of quantification (LOQ) and standard deviations (SD) are given in Table 4. All data are reported on a wet weight basis.
Both matrices, bream liver and SPM, could be successfully validated at the indicated LOQ levels. These levels range in a substance, but also in a matrix-dependent manner from 0.2 to 2.0 μg kg−1, and reflect the lowest achievable values according to observations derived from the matrix calibration functions shown in Fig. S1 of the Electronic supplementary material (ESM). The recoveries are within 90–110% and the relative standard deviation (RSD) is ≤ 10% (n = 6). The only exception is chlorophacinone whose mean recovery is 116% and RSD 26.3% in SPM. This seems acceptable since no quantitative data are being reported for chlorophacinone in this study. The achieved LOQs are similar or lower than recently published LC-MS/MS-based multi-methods for AR in tissues, ranging from 0.9 to 250 μg kg−1 (Fourel et al. 2017a, Hernandez et al. 2013, Jin et al. 2009, Marek and Koskinen 2007, Marsalek et al. 2015, Smith et al. 2017, Vandenbroucke et al. 2008b).
Analysis of temporal trends
Temporal trends for brodifacoum in fish tissue (wet weight data) were analyzed by applying a software tool from the German Environment Agency (LOESS-Trend, Version 1.1, based on Microsoft Excel). The application fits a locally weighted scatterplot smoother (LOESS) with a fixed window width of 7 years through the annual rodenticide levels. Then, tests on the significance of linear and non-linear trend components are conducted by means of an analysis of variance (ANOVA) following the procedure of Fryer and Nicholson (1999). For years with analytical results less than the LOQ, the data gaps were treated as ½ LOQ values.
Results and discussion
Results of environmental analysis
Spatial comparison: measurement of samples from different ESB sampling sites of the years 2011 and 2015
Results of the spatial monitoring exercise are presented in Table S1 (2011) and Table S6 (2015) in the electronic supplemental material and are summarized in Fig. 1 (for 2015) along with the spatial distribution of the sampling sites across Germany. Within all bream liver samples, only SGARs were found above the LOQ. For the year 2015, brodifacoum was the major AR found. It was detected in 88% of the samples with a maximum concentration of 12.5 μg kg−1 (average (Ø) 3.4 μg kg−1 and median 2.1 μg kg−1). Difenacoum was found in 44% of the samples at comparably lower concentrations of up to 0.7 μg kg−1 (Ø 0.1 μg kg−1). Bromadiolone was found in 17% of the samples at peaks of 7.1 μg kg−1 with Ø of 0.6 μg kg−1, difethialone in 6% with highest levels at 6.3 μg kg−1, and flocoumafen in 12% at highest levels of 0.3 μg kg−1. For 2011, quite different substance and concentration patterns were found, as presented in Table S1, which may be due to the seasonal character of substance usage and varying intervals between application and sampling. To our knowledge, this is the first evidence of AR in freshwater fish tissue.
In addition, a set of co-located suspended particulate matter (SPM) samples from the year 2015 were analyzed. The results are presented in Table S3 and also included in Fig. 1. In contrast to results of the bream liver samples, in SPM, only bromadiolone was found above LOQ in 56% of the 16 samples with highest values of 9.2 μg kg−1 (Ø 4.9 μg kg−1; median 4.3 μg kg−1).
No ARs were detected > the LOQ in the five otter livers that were analyzed in addition to bream and SPM to exemplary include a fish-eating mammal as a top predator in the food web in this study. In contrast, in a study using French otter samples from 2010, 10% of the tested otter samples were contaminated with bromadiolone at levels of 400 and 850 μg kg−1 fresh weight (Lemarchand et al. 2010).
Temporal trend analysis: retrospective monitoring for rodenticides in fish liver samples from Saar River/Rehlingen and Elbe River/Prossen
Based on the results of the 2011 and 2015 spatial analysis, the sampling sites in Rehlingen at the Saar River and Prossen at the Elbe River were chosen for the temporal analysis.
The results of the temporal analysis are summarized in S4 (Saar/Rehlingen) and Table S5 (Elbe/Prossen).
From these temporal data, a significant time trend could be drawn only for brodifacoum at Saar/Rehlingen (Fig. 2). This trend indicated an average increase of brodifacoum at 0.3 μg kg−1 per year for the observed period and 1.3 μg kg−1 per year for the last 7 years.
Time trend analysis of both sampling sites for brodifacoum using the LOESS-Trend tool (compare “Materials and methods” section). Circles reflect actual results (mean values of replicates of pooled fish samples), while the blue solid or dashed line reflects the linear fit, the green dashed line the dynamic fit, and the gray area the confidence interval (α = 0.05). For mean value calculations, data below the LOQ were substituted by a concentration of 50% of the LOQ (LOQ = 1.0 μg kg−1 for brodifacoum; compare Table S4 and S5 in the ESM). “-” indicates values results below LOQ
Notably, brodifacoum was the most abundant AR measured in fish from both locations. Concentrations ranged between about 1 and 13 μg kg−1 in fish from Rehlingen and between 4 and 12 μg kg−1 in fish from Prossen, where it was found below LOQ only in the years 1992 and 2009. At Rehlingen also, bromadiolone, difenacoum, flocoumafen, and difethialone were found occasionally and at comparably low levels. Interestingly, for both sampling sites, the diversity of detected AR was higher in 2015 than in the years before. SPM was not subject to a retrospective analysis.
Assessment of relevance of rodenticide residues in fish and SPM
The analysis of fish samples at the different ESB sampling sites revealed the detectable occurrence in the order brodifacoum, difenacoum, bromadiolone, difethialone, and flocoumafen at levels above the LOQ. In contrast, in SPM, only bromadiolone was detectable.
Only SGARs were found above LOQ of the ARs measured in this study. This could be related to the higher persistency and potential for bioaccumulation of SGAR in comparison to FGAR. The partition coefficients n-octanol/water (log Kow), as a measure for lipophilicity and bioaccumulation potential, for FGARs are < 5 (at environmentally relevant pH) while the respective values for SGARs are all > 5 (at environmentally relevant pH). Also, available toxicity studies with rats show much shorter half-lives of FGAR in livers when compared to SGAR which may indicate faster elimination rates in target and non-target organisms (Daniels 2013). Another plausible reason for the lack of FGARs in the analyzed fish samples might be that FGARs are generally used less frequently than SGARs, especially for the control of rats in sewer systems, which is assumed to be the source of emissions to surface water bodies. A survey of 508 local municipal authorities in Germany responsible for the rat control in sewers (Krüger and Solas 2010) indicated that bromadiolone followed by difenacoum and brodifacoum were used most often by local authorities for the control of brown rats in sewer systems.
Comparison with bioconcentration factors, adsorption coefficients, and use patterns
The bioconcentration factors (BCF, the ratio of a substance concentration in water and in fish tissue and expressed as L kg−1) of fish as stated in the respective public Assessment Reports for their approval under BPR decrease in the following order: difethialone (39,974; estimated), brodifacoum (35,645; estimated), flocoumafen (24,300; measured), bromadiolone (460; measured), chlorophacinone (22.75; estimated), warfarin (≤ 21.6; measured), coumatetralyl (11.4; measured) (ECHA 2017a). The BCF values may explain why SGARs were detectable in the ESB fish samples, while FGARs were not. The organic carbon adsorption coefficients Koc [L kg−1], as given in the respective Assessment Reports for each of the active substances, increase in the order of warfarin (174), coumatetralyl (258), brodifacoum (9155), bromadiolone (14,770), chlorophacinone (75,800), flocoumafen (101,648), difenacoum (1.8* 106), difethialone (about 108) (ECHA 2017a). According to this, other highly adsorptive anticoagulants such as difenacoum, which is according to Krüger and Solas (2010) commonly used for sewer baiting in Germany, should also be expected to absorb to SPM (assumed that comparable amounts are emitted). There may be several reasons why this is not the case: SPM samples in the ESB archive were pooled samples of 12 monthly sub-samples, whereas only one ESB fish sample was collected per year after spawning at each of the riverine sampling sites. Depending on a seasonal exposure, higher or lower findings, compared to the concentrations actually found in this study, may be expected in SPM (e.g., when exposed in spring after treatment campaigns in municipal rodent control, AR can be expected in SPM), but the occurrence in fish that are sampled in a different season compared to the treatment might be unlikely, especially for FGAR with a low bioaccumulation potential. However, this cannot fully explain the exclusive presence of bromadiolone and we are unclear why other ARs were not detected in this matrix.
The varying treatment lengths, intervals, and substance patterns of AR treatment campaigns in Germany may also help to explain the occasional detections of other SGAR, as their presence may reflect the major AR applied in the catchment that year. Data on the amounts of AR that were used are unfortunately rarely available (Pohl et al. 2015). Rodenticides, which have been used most often by municipal authorities for sewer baiting (Krüger and Solas 2010), were those found most frequently in fish (difenacoum, brodifacoum, and bromadiolone) and bromadiolone in SPM.
Rough estimations suggest that the AR concentrations detected in fish are plausible given the available data on concentrations in sewage treatment plant (STP) effluent (Gomez-Canela et al. 2014) and the known BCF of the detected compounds in fish (For details, see ESM).
An important aspect of rodenticides was recently identified to be the metabolism by Fourel et al. (2017b). They found a high abundance of trans-bromadiolone in red kite, indicating individual metabolic rates for the two bromadiolone enantiomers. If fish could also metabolize bromadiolone isomers selectively, this could explain why we found bromadiolone more frequently in SPM compared to fish liver. This does, in turn, not help to understand why other ARs were not found in SPM.
Synopsis
In summary, our findings demonstrate that contamination of wildlife with anticoagulant rodenticides, especially SGARs, also involves aquatic species and is not confined to predatory birds or mammals of the terrestrial food web. We detected residues of SGARs in fish samples from almost every ESB sampling site, including the rivers Rhine, Elbe, and Danube. The ubiquitous exposure of fish is in contrast to the rather low concentrations of SGARs in biocidal products which ranged from 25 mg kg−1 (difethialone) to 75 mg kg−1 (difenacoum). An amount of approximately 50 kg of anticoagulant rodenticide active substance is used annually for rat control in sewers and above ground by municipal authorities in Germany, with approximately 75% were used exclusively for sewer baiting (Krüger and Solas 2010). Given this relatively moderate amount of use, the prevalence of detectable rodenticide residues in fish samples appears surprisingly high. Whether this is entirely accounted for by the persistent and bioaccumulative properties of the SGARs requires investigation. In general, there remains a lack of understanding about both the impacts of rodenticides on aquatic life and the pathways by which these compounds enter the environment. There are few published data on rodenticide levels in waste water (Gomez-Canela et al. 2014) or surface water and no information on what specific substances or amounts are used. Experimentally derived BCF values for ARs are not always available and modeled BCF value may not enable a sound assessment of the potential for bioaccumulation in fish. Therefore, it is important to generate a better overview on the temporal spatial occurrence of AR in freshwater environments and to identify relevant sources and entry pathways. Further research is needed to unravel the exposure of freshwater environments to rodenticides. This may involve environmental fate studies as well as additional spatial and temporal monitoring activities. Monitoring of AR can thereby provide additional key information for their environmental risk assessment and the need to set appropriate risk mitigation measures within their authorization as biocides in the European Union.
References
Alomar H, Chabert A, Coeurdassier M, Vey D, Berny P (2018) Accumulation of anticoagulant rodenticides (chlorophacinone, bromadiolone and brodifacoum) in a non-target invertebrate, the slug, Deroceras reticulatum. Sci Total Environ 610:576–582
BAuA (2017) List of biocidal products authorized in Germany in product type 14 (rodenticides). Accessed Sept. 2017. Federal Institute for Occupational Safety and Health (BAuA)
Berny PJ, Buronfosse T, Buronfosse F, Lamarque F, Lorgue G (1997) Field evidence of secondary poisoning of foxes (Vulpes vulpes) and buzzards (Buteo buteo) by bromadiolone, a 4-year survey. Chemosphere 35(8):1817–1829. https://doi.org/10.1016/S0045-6535(97)00242-7
Bidny S, Gago K, David M, Duong T, Albertyn D, Gunja N (2015) A validated LC-MS-MS method for simultaneous identification and quantitation of rodenticides in blood. J Anal Toxicol 39(3):219–224. https://doi.org/10.1093/jat/bku175
Buckle AP, Eason CT (2015) Control methods: chemical. In: Buckle A, Smith R (editors), Rodent pests and their control. CABI Book, pp. 123. https://doi.org/10.1079/9781845938178.0000
BVL (2017) Register of plant protection products. BVL: The Federal Office for Consumer Protection and Food Safety, Germany
Chen XH, Cai MQ, Ouyang XK, Jin MC (2009) Ion chromatography tandem mass spectrometry for simultaneous confirmation and determination of indandione rodenticides in serum. Biomed Chromatogr 23(11):1217–1226. https://doi.org/10.1002/bmc.1246
Christensen TK, Lassen P, Elmeros M (2012) High exposure rates of anticoagulant rodenticides in predatory bird species in intensively managed landscapes in Denmark. Arch Environ Contam Toxicol 63:437–444
Daniels D 2013: Memorandum: second generation anticoagulant rodenticide assessment, Department of Pesticide Regulation, Sacramento, California, http://www.cdpr.ca.gov/docs/registration/reevaluation/chemicals/brodifacoum_final_assess.pdf
Dowding CV, Shore RF, Worgan A, Baker PJ, Harris S (2010) Accumulation of anticoagulant rodenticides in a non-target insectivore, the European hedgehog (Erinaceus europaeus). Environ Pollut 158(1):161–166. https://doi.org/10.1016/j.envpol.2009.07.017
Eason CT, Murphy EC, Wright GR, Spurr EB (2002) Assessment of risks of brodifacoum to non-target birds and mammals in New Zealand. Ecotoxicology 11(1):35–48. https://doi.org/10.1023/A:1013793029831
ECHA (2007) Difethialone: assessment report product-type 14 (rodenticides). Annex I. 21 June 2007. Rapporteur Member State: Norway. European Chemicals Agency. https://circabc.europa.eu/sd/a/8d235346-557e-4906-ad4b-86714378e8ed/Difethialone%20assessment%20report%20as%20finalised%20on%2021.06.07.pdf
ECHA (2010) Assessment report bromadiolone product-type 14 (rodenticides) Directive 98/8/EC concerning the placing of biocidal products on the market. Inclusion of active substances in annex I or IA to Directive 98/8/EC. European Chemicals Agency, Sweden. https://circabc.europa.eu/sd/a/861933f1-29f7-4758-8d69-7d9eafea4ca5/Assessment%20Report%20revised%2016122011.pdf
ECHA (2017a) Biocidal active substances. European_Chemicals_Agency. https://echa.europa.eu/information-on-chemicals/biocidal-active-substances
ECHA (2017b) Biocidal products R4BP3. European_Chemicals_Agency, Helsinki, Finland. https://echa.europa.eu/information-on-chemicals/biocidal-products
Elliott JE, Hindmarch S, Albert CA, Emery J, Mineau P, Maisonneuve F (2014) Exposure pathways of anticoagulant rodenticides to nontarget wildlife. Environ Monit Assess 186(2):895–906. https://doi.org/10.1007/s10661-013-3422-x
Elmeros M, Christensen TK, Lassen P (2011) Concentrations of anticoagulant rodenticides in stoats Mustela erminea and weasels Mustela nivalis from Denmark. Sci Total Environ 409(12):2373–2378. https://doi.org/10.1016/j.scitotenv.2011.03.006
Elmeros M, Lassen P, Bossi R, Topping CJ (2018) Exposure of stone marten (Martes foina) and polecat (Mustela putorius) to anticoagulant rodenticides: effects of regulatory restrictions of rodenticide use. Sci Total Environ 612:1358–1364. https://doi.org/10.1016/j.scitotenv.2017.09.034
Fisher P, Funnell E, Fairweather A, Brown L, Campion M (2012) Accidental discharge of brodifacoum baits into a freshwater lake: a case study. B Environ Contam Tox 88(2):226–228. https://doi.org/10.1007/s00128-011-0470-1
Fourel I, Damin-Pernik M, Benoit E, Lattard V (2017a) Core-shell LC-MS/MS method for quantification of second generation anticoagulant rodenticides diastereoisomers in rat liver in relationship with exposure of wild rats. J Chromatogr B Analyt Technol Biomed Life Sci 1041-1042:120–132. https://doi.org/10.1016/j.jchromb.2016.12.028
Fourel I, Damin-Pernik M, Benoit E, Lattard V (2017b) Cis-bromadiolone diastereoisomer is not involved in bromadiolone red kite (Milvus milvus) poisoning. Sci Total Environ 601-602:1412–1417. https://doi.org/10.1016/j.scitotenv.2017.06.011
Fournier-Chambrillon C, Berny PJ, Coiffier O, Barbedienne P, Dasse B, Delas G, Galineau H, Mazet A, Pouzenc P, Rosoux R, Fournier P (2004) Evidence of secondary poisoning of free-ranging riparian mustelids by anticoagulant rodenticides in France: implications for conservation of European mink (Mustela lutreola). J Wildl Dis 40(4):688–695. https://doi.org/10.7589/0090-3558-40.4.688
Fryer RJ, Nicholson MD (1999) Using smoothers for comprehensive assessments of contaminant time series in marine biota. ICES J Mar Sci 56(5):779–790. https://doi.org/10.1006/jmsc.1999.0499
Geduhn A, Esther A, Schenke D, Mattes H, Jacob J (2014) Spatial and temporal exposure patterns in non-target small mammals during brodifacoum rat control. Sci Total Environ 496:328–338
Geduhn A, Jacob J, Schenke D, Keller B, Kleinschmidt S, Esther A (2015) Relation between intensity of biocide practice and residues of anticoagulant rodenticides in red foxes (Vulpes vulpes). PLoS One 10(9):e0139191. https://doi.org/10.1371/journal.pone.0139191
Geduhn A, Esther A, Schenke D, Gabriel D, Jacob J (2016) Prey composition modulates exposure risk to anticoagulant rodenticides in a sentinel predator, the barn owl. Sci Total Environ 544:150–157
Gomez-Canela C, Barata C, Lacorte S (2014) Occurrence, elimination, and risk of anticoagulant rodenticides and drugs during wastewater treatment. Environ Sci Pollut Res Int 21:7194–7203
Gomez-Ramirez P, Shore RF, van den Brink NW, van Hattum B, Bustnes JO, Duke G, Fritsch C, Garcia-Fernandez AJ, Helander BO, Jaspers V, Krone O, Martinez-Lopez E, Mateo R, Movalli P, Sonne C (2014) An overview of existing raptor contaminant monitoring activities in Europe. Environ Int 67:12–21. https://doi.org/10.1016/j.envint.2014.02.004
Hernandez AM, Bernal J, Bernal JL, Martin MT, Caminero C, Nozal MJ (2013) Analysis of anticoagulant rodenticide residues in Microtus arvalis tissues by liquid chromatography with diode array, fluorescence and mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci 925:76–85. https://doi.org/10.1016/j.jchromb.2013.02.032
Hughes J, Sharp E, Taylor MJ, Melton L, Hartley G (2013) Monitoring agricultural rodenticide use and secondary exposure of raptors in Scotland. Ecotoxicology 22:974–984
Imran M, Shafi H, Wattoo SA, Chaudhary MT, Usman HF (2015) Analytical methods for determination of anticoagulant rodenticides in biological samples. Forensic Sci Int 253:94–102. https://doi.org/10.1016/j.forsciint.2015.06.008
Jin MC, Chen XH, Zhu Y (2007) Determination of five 4-hydroxycoumarin rodenticides in animal liver tissues by ion chromatography with fluorescence detection. J Chromatogr A 1155(1):57–61. https://doi.org/10.1016/j.chroma.2006.12.074
Jin MC, Chen XH, Ye ML, Zhu Y (2008) Analysis of indandione anticoagulant rodenticides in animal liver by eluent generator reagent free ion chromatography coupled with electrospray mass spectrometry. J Chromatogr A 1213(1):77–82. https://doi.org/10.1016/j.chroma.2008.08.100
Jin MC, Cai MQ, Chen XH (2009) Simultaneous measurement of indandione-type rodenticides in human serum by liquid chromatography-electrospray ionization- tandem mass spectrometry. J Anal Toxicol 33(6):294–300. https://doi.org/10.1093/jat/33.6.294
Klein R, Paulus M, Tarricone K, Teubne D (2012) Guidelines for sampling, transport, storage and chemical characterization of environmental and human samples, guideline for sampling and sample treatment bream (Abramis brama); V 2.0.2. German Environmental Agency
Krüger G, Solas H (2010) Neighbours in the sewer network results of a questionnaire survey on rat control. Korrespondenz Abwasser Abfall 57:430–435
Langford KH, Reid M, Thomas KV (2013) The occurrence of second generation anticoagulant rodenticides in non-target raptor species in Norway. Sci Total Environ 450-451:205–208. https://doi.org/10.1016/j.scitotenv.2013.01.100
Larsen J (2003) Supplement to the methodology for risk evaluation of biocides. Emission scenario document for biocides used as rodenticides: CA-Jun03-Doc.8.2-PT14. In: EPA D (Hrsg). https://echa.europa.eu/documents/10162/16908203/pt14_rodenticides_en.pdf/159a8bb4-69bb-4bc4-9267-0b3221d16d09
Lemarchand C, Rosoux R, Berny P (2010) Organochlorine pesticides, PCBs, heavy metals and anticoagulant rodenticides in tissues of Eurasian otters (Lutra lutra) from upper Loire River catchment (France). Chemosphere 80:1120–1124
Marek LJ, Koskinen WC (2007) Multiresidue analysis of seven anticoagulant rodenticides by high-performance liquid chromatography/electrospray/mass spectrometry. J Agric Food Chem 55(3):571–576. https://doi.org/10.1021/jf061440y
Marsalek P, Modra H, Doubkova V, Vecerek V (2015) Simultaneous determination of ten anticoagulant rodenticides in tissues by column-switching UHPLC-ESI-MS/MS. Anal Bioanal Chem 407:7849. https://doi.org/10.1007/s00216-015-8954-1
Masuda BM, Fisher P, Beaven B (2015) Residue profiles of brodifacoum in coastal marine species following an island rodent eradication. Ecotoxicol Environ Saf 113:1–8
McDonald RA, Harris S, Turnbull G, Brown P, Fletcher M (1998) Anticoagulant rodenticides in stoats (Mustela erminea) and weasels (Mustela nivalis) in England. Environ Pollut 103:17–23
McMillin S, Hosea R, Finlayson B, Cypher B, Mekebri A (2008): Anticoagulant rodenticide exposure in an urban population of the San Joaquin kit fox, Proceedings of the Twenty-Third Vertebrate Pest Conference, pp. 163–165
Newton I, Wyllie I, Freestone P (1990) Rodenticides in British barn owls. Environ Pollut 68:101–117
Nogeire TM, Lawler JJ, Schumaker NH, Cypher BL, Phillips SE (2015) Land use as a driver of patterns of rodenticide exposure in modeled kit fox populations. PLoS One 10(8):e0133351. https://doi.org/10.1371/journal.pone.0133351
Pohl P, Dulio V, Botta F, Schwarzbauer J, Rüdel H (2015) Environmental monitoring of biocides in Europe—compartment-specific strategies, NORMAN/UBA Workshop. (FKZ) 3712 67 403. German Environment Agency, Dessau Rosslau
Primus T, Wright G, Fisher P (2005) Accidental discharge of brodifacoum baits in a tidal marine environment: a case study. B Environ Contam Tox 74(5):913–919. https://doi.org/10.1007/s00128-005-0668-1
Proulx G, MacKenzie N (2012) Relative abundance of American badger (Taxidea taxus) and red fox (Vulpes vulpes) in landscapes with high and low rodenticide poisoning levels. Integr Zool 7(1):41–47. https://doi.org/10.1111/j.1749-4877.2011.00276.x
Quinn JH, Girard YA, Gilardi K, Hernandez Y, Poppenga R, Chomel BB, Foley JE, Johnson CK (2012) Pathogen and rodenticide exposure in American badgers (Taxidea taxus) in California. J Wildl Dis 48(2):467–472. https://doi.org/10.7589/0090-3558-48.2.467
Rattner BA, Lazarus RS, Elliott JE, Shore RF, van den Brink N (2014) Adverse outcome pathway and risks of anticoagulant rodenticides to predatory wildlife. Environ Sci Technol 48(15):8433–8445. https://doi.org/10.1021/es501740n
Rattner BA, Horak KE, Lazarus RS, Schultz SL, Knowles S, Abbo BG, Volker SF (2015) Toxicity reference values for chlorophacinone and their application for assessing anticoagulant rodenticide risk to raptors. Ecotoxicology 24(4):720–734. https://doi.org/10.1007/s10646-015-1418-8
Ricking M, Winkler A, Schneider M (2012): Guidelines for sampling, transport, storage and chemical characterization of environmental and human samples, guideline for sampling and sample treatment suspended particulate matter; V 4.0.2. German Environmental Agency
Ruiz-Suarez N, Henriquez-Hernandez LA, Valeron PF, Boada LD, Zumbado M, Camacho M, Almeida-Gonzalez M, Luzardo OP (2014) Assessment of anticoagulant rodenticide exposure in six raptor species from the Canary Islands (Spain). Sci Total Environ 485-486:371–376. https://doi.org/10.1016/j.scitotenv.2014.03.094
Ruiz-Suarez N, Melero Y, Giela A, Henriquez-Hernandez LA, Sharp E, Boada LD, Taylor MJ, Camacho M, Lambin X, Luzardo OP, Hartley G (2016) Rate of exposure of a sentinel species, invasive American mink (Neovison vison) in Scotland, to anticoagulant rodenticides. Sci Total Environ 569-570:1013–1021. https://doi.org/10.1016/j.scitotenv.2016.06.109
Schaff JE, Montgomery MA (2013) An HPLC-HR-MS-MS method for identification of anticoagulant rodenticides in blood. J Anal Toxicol 37(6):321–325. https://doi.org/10.1093/jat/bkt036
Shore RF, Birks JD, Afsar A, Wienburg CL, Kitchener AC (2003) Spatial and temporal analysis of second-generation anticoagulant rodenticide residues in polecats (Mustela putorius) from throughout their range in Britain, 1992-1999. Environ Pollut 122(2):183–193. https://doi.org/10.1016/S0269-7491(02)00297-X
Smith LL, Liang B, Booth MC, Filigenzi MS, Tkachenko A, Gaskill CL (2017) Development and validation of quantitative ultraperformance liquid chromatography-tandem mass spectrometry assay for anticoagulant rodenticides in liver. J Agric Food Chem 65(31):6682–6691. https://doi.org/10.1021/acs.jafc.7b02280
Stansley W, Cummings M, Vudathala D, Murphy LA (2014) Anticoagulant rodenticides in red-tailed hawks, Buteo jamaicensis, and great horned owls, Bubo virginianus, from New Jersey, USA, 2008-2010. Bull Environ Contam Toxicol 92(1):6–9. https://doi.org/10.1007/s00128-013-1135-z
Thomas PJ, Mineau P, Shore RF, Champoux L, Martin PA, Wilson LK, Fitzgerald G, Elliott JE (2011) Second generation anticoagulant rodenticides in predatory birds: probabilistic characterisation of toxic liver concentrations and implications for predatory bird populations in Canada. Environ Int 37(5):914–920. https://doi.org/10.1016/j.envint.2011.03.010
Tosh DG, McDonald RA, Bearhop S, Lllewellyn NR, Fee S, Sharp EA, Barnett EA, Shore RF (2011) Does small mammal prey guild affect the exposure of predators to anticoagulant rodenticides? Environ Pollut 159(10):3106–3112. https://doi.org/10.1016/j.envpol.2011.03.028
Vandenbroucke V, Bousquet-Melou A, De Backer P, Croubels S (2008a) Pharmacokinetics of eight anticoagulant rodenticides in mice after single oral administration. J Vet Pharmacol Ther 31(5):437–445. https://doi.org/10.1111/j.1365-2885.2008.00979.x
Vandenbroucke V, Desmet N, De Backer P, Croubels S (2008b) Multi-residue analysis of eight anticoagulant rodenticides in animal plasma and liver using liquid chromatography combined with heated electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 869(1-2):101–110. https://doi.org/10.1016/j.jchromb.2008.05.011
Walker LA, Turk A, Long SM, Wienburg CL, Best J, Shore RF (2008) Second generation anticoagulant rodenticides in tawny owls (Strix aluco) from Great Britain. Sci Total Environ 392(1):93–98. https://doi.org/10.1016/j.scitotenv.2007.10.061
Zhu L, Yan H, Shen B, Shi Y, Shen M, Xiang P (2013) Determination of bromadiolone and brodifacoum in human hair by liquid chromatography/tandem mass spectrometry and its application to poisoning cases. Rapid Commun Mass Spectrom 27(4):513–520. https://doi.org/10.1002/rcm.6477
Acknowledgements
The authors would like to thank all current and previous partners of the German ESB program for their long-term commitment in providing high quality sample material. The fish sampling was organized by Biogeography, Trier University, Germany, and the SPM sampling was conducted by Department Earth Sciences, Anthropocene Research, Geochemistry, Freie Universität Berlin, Germany. The authors are grateful to Olaf Zinke, Department of Zoology at the Museum of Western Lusatia in Kamenz, for providing the otter samples and Georg Radermacher, Fraunhofer IME, for the preparation of the otter samples. Jörg Wellmitz (Umweltbundesamt) is acknowledged for kindly providing the LOESS-Trend software tool for trend evaluations.
Funding
The funding of the project by the German Environment Agency (FKZ 3712 67 403 and ESB program) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible editor: Ester Heath
Highlights
• For the first time, anticoagulant rodenticides were identified in freshwater fish and SPM.
• A multi-method was developed to capture eight different anticoagulant rodenticides.
• Second generation anticoagulant rodenticides were found at levels > 10 μg kg−1.
• A differing distribution of rodenticides between fish and SPM was found.
• At one site, the temporal trend of brodifacoum increased significantly in bream liver.
Electronic supplementary material
ESM 1
(DOCX 187 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kotthoff, M., Rüdel, H., Jürling, H. et al. First evidence of anticoagulant rodenticides in fish and suspended particulate matter: spatial and temporal distribution in German freshwater aquatic systems. Environ Sci Pollut Res 26, 7315–7325 (2019). https://doi.org/10.1007/s11356-018-1385-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1385-8