Abstract
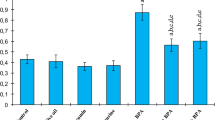
Bisphenol A (BPA) is a widely used environmental pollutant in the production of plastics but causes hepatotoxicity in mammals. In the present study, we studied the BPA-induced oxidative stress in rats and ameliorative potential of Adiantum capillus-veneris L. plant. It was concluded that the BPA can reduce the body and liver weight, increase in biochemical levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), total bilirubin, and disturb the normal hepatic physiology, histology, and metabolism. Additionally, liver histology shows hepatic necrosis, congestion, and vacuolization in exposed individuals. In contrast, simultaneous exposure of A. capillus-veneris and BPA showed declining trend in serum biomarker levels and normal histopathological structures. We conclude that the A. capillus-veneris plant is antioxidant in nature and can reduce the BPA-induced toxicity. These findings are very helpful to understand the BPA-induced hepatic toxicity and ameliorative potential of A. capillus-veneris plant and are of great importance in risk assessment of xenobiotics.



Similar content being viewed by others
References
Abbasi AM, Shah MH, Li T, Fu X, Guo X, & Liu, RH (2015) Ethnomedicinal values, phenolic contents and antioxidant properties of wild culinary vegetables. J Ethnopharmacol 162:333-345
Ahmed A, Wadud A, Jahan N, Bilal A, Hajera S (2013) Efficacy of Adiantum capillus veneris Linn in chemically induced urolithiasis in rats. J Ethnopharmacol 146(1):411–416. https://doi.org/10.1016/j.jep.2013.01.011
Ahmed W et al (2015) Bisphenol A toxicity in adult male rats: hematological, biochemical and histopathological approach. Glob Veternaria 14:228–238
Akingbemi BT (2005) Estrogen regulation of testicular function. Reprod Biol Endocrinol 3(1):51. https://doi.org/10.1186/1477-7827-3-51
Al-Hiyasat AS, Darmani H, Elbetieha AM (2002) Effects of bisphenol A on adult male mouse fertility. Eur J Oral Sci 110(2):163–167. https://doi.org/10.1034/j.1600-0722.2002.11201.x
Angulo P, Keach JC, Batts KP, Lindor KD (1999) Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 30(6):1356–1362. https://doi.org/10.1002/hep.510300604
Ansari R, & Ekhlasi-Kazaj K (2012) Adiantum capillus-veneris. L: Phytochemical Constituents, Traditional Uses and Pharmacological Properties: A Review. J Adv Sci Res 3(4):15-20
Asahi J, Kamo H, Baba R, Doi Y, Yamashita A, Murakami D, Hanada A, Hirano T (2010) Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci 87(13):431–438. https://doi.org/10.1016/j.lfs.2010.08.007
Atcha Z, Rourke C, Neo AH, Goh CW, Lim JS, Aw CC, Browne ER, Pemberton DJ (2010) Alternative method of oral dosing for rats. J Am Assoc Lab Anim Sci 49(3):335–343
Bancroft JD, & Gamble M (2008) Theory and Practice of Histopathological Techniques. (6th ed.). Elsevier Health Sciences, London
Berkowitz G (2006) Limitations of a case–control study on bisphenol A (BPA) serum levels and recurrent miscarriage. Hum Reprod 21(2):565–566. https://doi.org/10.1093/humrep/dei335
Bindhumol V, Chitra KC, Mathur PP (2003) Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 188(2):117–124. https://doi.org/10.1016/S0300-483X(03)00056-8
Chen Z, Zuo X, He D, Ding S, Xu F, Yang H, Jin X, Fan Y, Ying L, Tian C, Ying C (2017) Long-term exposure to a ‘safe’dose of bisphenol A reduced protein acetylation in adult rat testes. Sci Rep 7:40337. https://doi.org/10.1038/srep40337
Chitra K et al (2003) Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology 185(1):119–127. https://doi.org/10.1016/S0300-483X(02)00597-8
Crookham JN, & Dapson RW (1991) Hazardous Chemicals in the Histopathology Laboratory: Regulations Risks Handling & Disposal. Anatech
Dekant W, Völkel W (2008) Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol 228(1):114–134. https://doi.org/10.1016/j.taap.2007.12.008
El Tawab AMA et al (2014) Protective effect of Satureja montana extract on cyclophosphamide-induced testicular injury in rats. Chem Biol Interact 224:196–205. https://doi.org/10.1016/j.cbi.2014.11.001
Frye C, Bo E, Calamandrei G, Calzà L, Dessì-Fulgheri F, Fernández M, Fusani L, Kah O, Kajta M, le Page Y, Patisaul HB, Venerosi A, Wojtowicz AK, Panzica GC (2012) Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol 24(1):144–159. https://doi.org/10.1111/j.1365-2826.2011.02229.x
Gangadharan L, Valenzuela MR (2001) Interrelationships between income, health and the environment: extending the Environmental Kuznets Curve hypothesis. Ecol Econ 36(3):513–531. https://doi.org/10.1016/S0921-8009(00)00250-0
Giboney PT (2005) Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician 71(6):1105–1110
Gurmeet KSS, Rosnah I, Normadiah MK, Das S, Mustafa AM (2014). Detrimental effects of bisphenol A on development and functions of the male reproductive system in experimental rats. EXCLI J 13:151
Haider S, Nazreen S, Alam MM, Gupta A, Hamid H, Alam MS (2011) Anti-inflammatory and anti-nociceptive activities of ethanolic extract and its various fractions from Adiantum capillus veneris Linn. J Ethnopharmacol 138(3):741–747. https://doi.org/10.1016/j.jep.2011.10.012
Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MH, AlOlayan EM (2012) Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxidative Med Cell Longev 2012:1–6. https://doi.org/10.1155/2012/194829
HIROI H, Tsutsumi O, Takeuchi T, Momoeda M, Ikezuki Y, OKamura A, Yokota H, Taketani Y (2004) Differences in serum bisphenol a concentrations in premenopausal normal women and women with endometrial hyperplasia. Endocr J 51(6):595–600. https://doi.org/10.1507/endocrj.51.595
Ho Y-S, Magnenat JL, Gargano M, Cao J (1998) The nature of antioxidant defense mechanisms: a lesson from transgenic studies. Environ Health Perspect 106(Suppl 5):1219–1228. https://doi.org/10.1289/ehp.98106s51219
Hussein RM, & Eid JI (2013) Pathological mechanisms of liver injury caused by oral administration of bisphenol A. Life Sci J 10(1)
Ibraheim ZZ, Ahmed AS, Gouda YG (2011) Phytochemical and biological studies of Adiantum capillus-veneris L. Saudi Pharm J 19(2):65–74. https://doi.org/10.1016/j.jsps.2011.01.007
Inoue H, Yokota H, Makino T, Yuasa A, Kato S (2001) Bisphenol A glucuronide, a major metabolite in rat bile after liver perfusion. Drug Metab Dispos 29(8):1084–1087
Inoue H, Yuki G, Yokota H, Kato S (2003) Bisphenol A glucuronidation and absorption in rat intestine. Drug Metab Dispos 31(1):140–144. https://doi.org/10.1124/dmd.31.1.140
Izzotti A, Longobardi M, Cartiglia C, D'Agostini F, Kanitz S, de Flora S (2010) Pharmacological modulation of genome and proteome alterations in mice treated with the endocrine disruptor bisphenol A. Curr Cancer Drug Targets 10(2):147–154. https://doi.org/10.2174/156800910791054220
Jayashree S, Indumathi D, Akilavalli N, Sathish S, Selvaraj J, Balasubramanian K (2013) Effect of bisphenol-A on insulin signal transduction and glucose oxidation in liver of adult male albino rat. Environ Toxicol Pharmacol 35(2):300–310. https://doi.org/10.1016/j.etap.2012.12.016
Kabuto H, Amakawa M, Shishibori T (2004) Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci 74(24):2931–2940. https://doi.org/10.1016/j.lfs.2003.07.060
Keshavan P, Schwemberger SJ, Smith DLH, Babcock GF, Zucker SD (2004) Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int J Cancer 112(3):433–445. https://doi.org/10.1002/ijc.20418
Kleiner DE, Brunt EM, van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research Network (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41(6):1313–1321. https://doi.org/10.1002/hep.20701
Korkmaz A, Ahbab MA, Kolankaya D, Barlas N (2010) Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem Toxicol 48(10):2865–2871. https://doi.org/10.1016/j.fct.2010.07.019
Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D (2008) Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300(11):1303–1310. https://doi.org/10.1001/jama.300.11.1303
Lee YJ, Ryu HY, Kim HK, Min CS, Lee JH, Kim E, Nam BH, Park JH, Jung JY, Jang DD, Park EY, Lee KH, Ma JY, Won HS, Im MW, Leem JH, Hong YC, Yoon HS (2008) Maternal and fetal exposure to bisphenol A in Korea. Reprod Toxicol 25(4):413–419. https://doi.org/10.1016/j.reprotox.2008.05.058
Marmugi A, Lasserre F, Beuzelin D, Ducheix S, Huc L, Polizzi A, Chetivaux M, Pineau T, Martin P, Guillou H, Mselli-Lakhal L (2014) Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology 325:133–143. https://doi.org/10.1016/j.tox.2014.08.006
Matsumoto J, Yokota H, Yuasa A (2002) Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Health Perspect 110(2):193–196. https://doi.org/10.1289/ehp.02110193
Morgan AM, el-Ballal SS, el-Bialy BE, el-Borai NB (2014) Studies on the potential protective effect of cinnamon against bisphenol A-and octylphenol-induced oxidative stress in male albino rats. Toxicol Rep 1:92–101. https://doi.org/10.1016/j.toxrep.2014.04.003
Mourad IM, Khadrawy YA (2012) The sensetivity of liver, kidney andtestis of rats to oxidative stress induced by different doses of bisphenol A. Life 50:19
Muñoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, Soto AM (2005) Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology 146(9):4138–4147. https://doi.org/10.1210/en.2005-0340
Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM (2007) Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol 23(3):383–390. https://doi.org/10.1016/j.reprotox.2006.10.002
Nandi D, Patra RC, Swarup D (2005) Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology 211(1):26–35. https://doi.org/10.1016/j.tox.2005.02.013
Paulose C, Dakshinamurti K (1987) Chronic catheterization using vascular-access-port in rats: blood sampling with minimal stress for plasma catecholamine determination. J Neurosci Methods 22(2):141–146. https://doi.org/10.1016/0165-0270(87)90008-2
Politch JA (2006) Bisphenol A and risk assessment. Environ Health Perspect 114(1):A16. https://doi.org/10.1289/ehp.114-a16a
Pourmorad F, Hosseinimehr SJ, & Shahabimajd N (2006) Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnol 5(11)
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63. https://doi.org/10.1093/ajcp/28.1.56
Richter CA et al (2007) In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 24(2):199–224
Rochester JR (2013) Bisphenol A and human health: a review of the literature. Reprod Toxicol 42:132–155. https://doi.org/10.1016/j.reprotox.2013.08.008
Sangai NP, Verma RJ (2011) Quercetin alleviates bisphenol A-induced changes in nucleic acid and protein contents in mice. Acta Pol Pharm Drug Res 68:867–873
Schuler P (1990) Natural antioxidants exploited commercially. Food antioxidants. Springer, Berlin, pp 99–170
Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S (2002) Stimulation of CD25+ CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol 3(2):135–142. https://doi.org/10.1038/ni759
Singh M, Singh N, Khare PB, Rawat AKS (2008) Antimicrobial activity of some important Adiantum species used traditionally in indigenous systems of medicine. J Ethnopharmacol 115(2):327–329. https://doi.org/10.1016/j.jep.2007.09.018
Snyder RW, Maness SC, Gaido KW, Welsch F, Sumner SCJ, Fennell TR (2000) Metabolism and disposition of bisphenol A in female rats. Toxicol Appl Pharmacol 168(3):225–234. https://doi.org/10.1006/taap.2000.9051
Strassburg C et al (2002) Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut 50(2):259–265. https://doi.org/10.1136/gut.50.2.259
Stump DG, Beck MJ, Radovsky A, Garman RH, Freshwater LL, Sheets LP, ... & Shiotsuka RN (2010) Developmental neurotoxicity study of dietary bisphenol A in Sprague-Dawley rats. Toxicol Sci 115(1):167-182
Sugiura-Ogasawara M, Ozaki Y, Sonta Si, Makino T, Suzumori K (2005) Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod 20(8):2325–2329. https://doi.org/10.1093/humrep/deh888
Swaroop K et al (2012) Influence of ethanolic leaf extract of Sargassum wightii and Adiantum capillus on histamine induced asthma in animal model. Int J Pharm Pharm Sci 4(4):121–123
Takeuchi T et al (2004) Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J 51(2):165–169. https://doi.org/10.1507/endocrj.51.165
Tan BL et al (2003) Assessment of pubertal development in juvenile male rats after sub-acute exposure to bisphenol A and nonylphenol. Toxicol Lett 143(3):261–270. https://doi.org/10.1016/S0378-4274(03)00172-3
Tyl R et al (2002) Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci 68(1):121–146. https://doi.org/10.1093/toxsci/68.1.121
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24(2):139–177. https://doi.org/10.1016/j.reprotox.2007.07.010
Yousaf B, Amina LG, Wang R, Qadir A, Ali MU, Kanwal Q, Munir B, Asmatullah AZ (2016) Bisphenol A exposure and healing effects of Adiantum capillus-veneris. Environ Sci Pollut Res 23(12):11645–11657. https://doi.org/10.1007/s11356-016-6330-0
Zade VS, et al (2013) Effect of aqueous extract of Moringa oleifera seed on sexual activity of male albino rats. Int J Bio Forum
Acknowledgements
Special thanks are given to the laboratory assistants for their help during experiment and analyses.
Funding
The authors greatly acknowledged the University of the Punjab for financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol of the study was approved by the departmental ethical committee of animal experiments, College of Earth and Environmental Sciences (CEES), University of the Punjab.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kanwal, Q., Qadir, A., Amina et al. Healing potential of Adiantum capillus-veneris L. plant extract on bisphenol A-induced hepatic toxicity in male albino rats. Environ Sci Pollut Res 25, 11884–11892 (2018). https://doi.org/10.1007/s11356-018-1211-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1211-3