Abstract
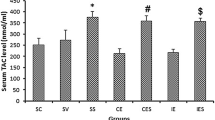
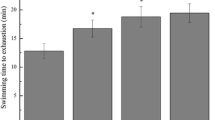
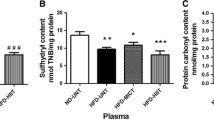
The current study examined fluctuations in oxidative stress markers following endurance training (ET) and consumption of purslane seeds (Ps) in rats after receiving H2O2. Fifty-four adult male Wistar rats were assigned to nine experimental groups: (1) control (intoxicated-no treatment); (2) ET; (3) ET + Ps 50 mg/kg/day; (4) ET + Ps 200 mg/kg/day; (5) ET + Ps 400 mg/kg/day; (6) Ps 50 mg/kg/day; (7) Ps 200 mg/kg/day; (8) Ps 400 mg/kg/day; (9) control (non-intoxicated, intact). The first eight groups were given 100 mg/kg of H2O2 to induce oxidative stress. Groups 2–5 were given ET for a period of 8 weeks. Heart and lung tissues were then exposed to evaluate the oxidative stress markers. Catalase, glutathione peroxidase, malondialdehyde, and superoxide dismutase enzymes were measured using ELISA kits. A marked improvement in enzyme concentration was observed in both tissues. It was more pronounced in the groups receiving higher doses of Ps + ET. The findings provide evidence that purslane seed supplementation has antioxidant potential alongside endurance training and improved the ability to cope with oxidative stress.




Similar content being viewed by others
References
Nita M, Grzybowski A (2016) The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev 2016:3164734
Borchi E, Bargelli V, Stillitano F, Giordano C, Sebastiani M, Nassi PA, d’Amati G, Cerbai E, Nediani C (2010) Enhanced ROS production by NADPH oxidase is correlated to changes in antioxidant enzyme activity in human heart failure. Biochim Biophys Acta 1802:331–338
Barber SC, Shaw PJ (2010) Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med 48:629–641
Cachofeiro V, Goicochea M, De Vinuesa SG, Oubiña P, Lahera V, Luño J (2008) Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease: new strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int 74:S4–S9
Dieterich S, Bieligk U, Beulich K, Hasenfuss G, Prestle J (2000) Gene expression of antioxidative enzymes in the human heart: increased expression of catalase in the end-stage failing heart. Circulation 101:33–39
Kao MP, Ang DS, Pall A, Struthers AD (2010) Oxidative stress in renal dysfunction: mechanisms, clinical sequelae and therapeutic options. J Hum Hypertens 24:1–8
Gomez-Cabrera MC, Domenech E, Vina J (2008) Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44:126–131
Powers SK, Nelson WB, Hudson MB (2011) Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med 51:942–950
Radak Z, Chung HY, Koltai E, Taylor AW, Goto S (2008) Exercise, oxidative stress and hormesis. Ageing Res Rev 7:34–42
Rahimi R (2017) Effect of resistance exercise on oxidative DNA damage and lipid peroxidation in trained and untrained men. Sport Sci Health 13:225–232
Georgakouli K, Manthou E, Fatouros IG, Georgoulias P, Deli CK, Koutedakis Y, Theodorakis Y, Jamurtas AZ (2017) Enhanced erythrocyte antioxidant status following an 8-week aerobic exercise training program in heavy drinkers. Alcohol, Fayetteville
Holbrook NJ, Ikeyama S (2002) Age-related decline in cellular response to oxidative stress: links to growth factor signaling pathways with common defects. Biochem Pharmacol 64:999–1005
Sacheck JM, Milbury PE, Cannon JG, Roubenoff R, Blumberg JB (2003) Effect of vitamin E and eccentric exercise on selected biomarkers of oxidative stress in young and elderly men. Free Radic Biol Med 34:1575–1588
Hollander J, Fiebig R, Gore M, Ookawara T, Ohno H, Ji L (2001) Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflügers Archiv 442:426–434
Arikawa AY, Thomas W, Gross M, Smith A, Phipps WR, Kurzer MS, Schmitz KH (2013) Aerobic training reduces systemic oxidative stress in young women with elevated levels of F2-isoprostanes. Contemp Clin Trials 34:212–217
Miyazaki H, Oh-ishi S, Ookawara T, Kizaki T, Toshinai K, Ha S, Haga S, Ji LL, Ohno H (2001) Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol 84:1–6
Jackson MJ, McArdle A (2011) Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J Physiol 589:2139–2145
Bouzid MA, Filaire E, McCall A, Fabre C (2015) Radical oxygen species, exercise and aging: an update. Sports Med 45:1245–1261
Dehghan F, Hajiaghaalipour F, Yusof A, Muniandy S, Hosseini SA, Heydari S, Salim LZA, Azarbayjani MA (2016) Saffron with resistance exercise improves diabetic parameters through the GLUT4/AMPK pathway in-vitro and in-vivo. Sci Rep 6:25139
Dehghan F, Soori R, Gholami K, Abolmaesoomi M, Yusof A, Muniandy S, Heidarzadeh S, Farzanegi P (2016) Purslane (Portulaca oleracea) seed consumption and aerobic training improves biomarkers associated with atherosclerosis in women with Type 2 diabetes (T2D). Sci Rep 6:37819
Lipinski B (2011) Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev 809696:9
Arshadi S, Azarbayjani MA, Hajaghaalipor F, Yusof A, Peeri M, Bakhtiyari S, Stannard RS, Osman NA, Dehghan F (2015) Evaluation of Trigonella foenum-graecum extract in combination with swimming exercise compared to glibenclamide consumption on type 2 diabetic rodents. Food Nutr Res 59:29717
Liu L, Howe P, Zhou YF, Xu ZQ, Hocart C, Zhan R (2000) Fatty acids and beta-carotene in australian purslane (Portulaca oleracea) varieties. J Chromatogr A 893:207–213
Uddin MK, Juraimi AS, Hossain MS, Nahar MA, Ali ME, Rahman MM (2014) Purslane weed (Portulaca oleracea): a prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci World J 2014:951019
Jouybari MF, Farzanegi P, Barari AR (2014) The effect of 8-week aerobic exercise with purslane supplementation consumption on peroxidant and antioxidants indicators in women with type 2 diabetes. J Shahid Sadoughi Univ Med Sci 22:928–939
Salehi A, Farzanegi P (2015) Effect of 8 weeks of resistance training with and without Portulacalo seeds on some of liver injury markers in women with diabetes type 2. Urmia Med J 25:968–978
Esmaillzadeh A, Zakizadeh E, Faghihimani E, Gohari M, Jazayeri S (2015) The effect of purslane seeds on glycemic status and lipid profiles of persons with type 2 diabetes: A randomized controlled cross-over clinical trial. J Res Med Sci 20:47–53
Ahmed OM, Hozayen WG, Sree HTA (2015) Effects of ethanolic purslane shoot and seed extracts on doxorubicin-induced hepatotoxicity in albino rats, vol 2. World Academy of Science, Engineering, Istanbul
El-Azime ASA, Hussein EM, Ashry OM (2014) Synergestic effect of aqueous purslane (Portulaca oleracea L.) extract and fish oil on radiation-induced damage in rats. Int J Radiat Biol 90:1184–1190
Garcia J, Martinez-Ballarin E, Robinson M, Allue J, Reiter R, Osuna C, Acuna-Castroviejo D (2000) Protective effect of beta-carbolines and other antioxidants on lipid peroxidation due to hydrogen peroxide in rat brain homogenates. Neurosci Lett 294:1–4
Mallikarjuna K, Shanmugam KR, Nishanth K, Wu MC, Hou CW, Kuo CH, Reddy KS (2010) Alcohol-induced deterioration in primary antioxidant and glutathione family enzymes reversed by exercise training in the liver of old rats. Alcohol (Fayetteville NY) 44:523–529
Samjoo I, Safdar A, Hamadeh M, Raha S, Tarnopolsky M (2013) The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutr Diabetes 3:e88
Steinbacher P, Eckl P (2015) Impact of oxidative stress on exercising skeletal muscle. Biomolecules 5:356–377
Goldfarb AH (1999) Nutritional antioxidants as therapeutic and preventive modalities in exercise-induced muscle damage. Can J Appl Physiol 24:249–266
Pal S, Chaki B, Chattopadhyay S, Bandyopadhyay A (2017) High intensity exercise induced oxidative stress and skeletal muscle damage in post-pubertal boys and girls: a comparative study. J Strength Cond Res 32:1045–1052
Bogdanis GC (2012) Effects of physical activity and inactivity on muscle fatigue. Front Physiol 3:142
Radak Z, Sasvari M, Nyakas C, Kaneko T, Tahara S, Ohno H, Goto S (2001) Single bout of exercise eliminates the immobilization-induced oxidative stress in rat brain. Neurochem Int 39:33–38
Gul M, Demircan B, Taysi S, Oztasan N, Gumustekin K, Siktar E, Polat MF, Akar S, Akcay F, Dane S (2006) Effects of endurance training and acute exhaustive exercise on antioxidant defense mechanisms in rat heart. Comp Biochem Physiol Part A Mol Integr Physiol 143:239–245
Kanter MM, Hamlin RL, Unverferth DV, Davis HW, Merola AJ (1985) Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol (Bethesda, Md: 1985) 59:1298–1303
Venditti P, Di Meo S (1996) Antioxidants, tissue damage, and endurance in trained and untrained young male rats. Arch Biochem Biophys 331:63–68
Gül M, Atalay M, Hänninen O (2003) Endurance training and glutathione-dependent antioxidant defense mechanism in heart of the diabetic rats. J Sports Sci Med 2:52
Somani S, Frank S, Rybak L (1995) Responses of antioxidant system to acute and trained exercise in rat heart subcellular fractions. Pharmacol Biochem Behav 51:627–634
Terblanche S (1999) The effects of exhaustive exercise on the activity levels of catalase in various tissues of male and female rats. Cell Biol Int 23:749–753
Bouzid MA, Hammouda O, Matran R, Robin S, Fabre C (2015) Influence of physical fitness on antioxidant activity and malondialdehyde level in healthy older adults. Appl Physiol Nutr Metab 40:582–589
Lu J, Borthwick F, Hassanali Z, Wang Y, Mangat R, Ruth M, Shi D, Jaeschke A, Russell JC, Field CJ, Proctor SD, Vine DF (2011) Chronic dietary n-3 PUFA intervention improves dyslipidaemia and subsequent cardiovascular complications in the JCR:LA-cp rat model of the metabolic syndrome. Br J Nutr 105:1572–1582
Nestel PJ (2000) Fish oil and cardiovascular disease: lipids and arterial function. Am J Clin Nutr 71:228S–231S
Oh JY (2010) Serum cystatin C as a biomarker for predicting coronary artery disease in diabetes. Korean Diabetes J 34:84–85
Jimoh F, Oladiji A (2005) Preliminary studies on Piliostigma thonningii seeds: proximate analysis, mineral composition and phytochemical screening. Afr J Biotechnol 4(12):1439–1442
Prakash D, Pal M (1991) Nutritional and antinutritional composition of vegetable and grain amaranth leaves. J Sci Food Agric 57:573–583
Miller T, Wing J, Huete A (1984) The agricultural potential of selected C4 plants in arid environments. J Arid Environ 7(3):275–286
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–1276
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have financial or other conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Soori, R., Shahedi, V., Akbarnejad, A. et al. Biochemical changes in oxidative stress markers following endurance training and consumption of purslane seed in rats with hydrogen peroxide-induced toxicity. Sport Sci Health 15, 133–139 (2019). https://doi.org/10.1007/s11332-018-0501-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-018-0501-y