Abstract
Purpose
Obstructive sleep apnea (OSA) is associated with renal impairs. As a novel pathophysiological hallmark of OSA, chronic intermittent hypoxia (CIH) enhances apoptosis and autophagy. The present study aims to evaluate the effect of telmisartan on CIH-induced kidney apoptosis and autophagy in a mouse model of OSA.
Materials and methods
Mice were randomly allocated to normoxia, CIH, and CIH+telmisartan groups (n = 12 in each group). The CIH exposure duration was 12 weeks. Mice in the CIH+telmisartan group received telmisartan administration. The terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay and western blotting of Bax and cleaved caspase-3 were conducted for evaluating apoptosis in kidney tissue. While the autophagy-related proteins, beclin-1 and LC3, were also observed via western blotting.
Results
The percentage of apoptotic cell in the CIH group was significantly higher than that of normoxia group; meanwhile, Bax and cleaved caspase-3 protein levels were increased in the CIH group than those of normoxia group (all p < 0.05). Compared with the normoxia group, mice in the CIH group had greater autophagy-related proteins (beclin-1 and LC3) expression. When compared to the CIH group, both the renal apoptosis and autophagy in the CIH+telmisartan group were decreased.
Conclusion
The CIH accelerates renal apoptosis and autophagy levels. Telmisartan ameliorating those levels suggests that it might prevent renal impairs from the CIH in OSA patients.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) is a highly prevalent medical disorder among middle-age adults [1]. Accumulated data confirmed the bidirectional association between kidney diseases and OSA; for one thing, the incidence and mortality of OSA in kidney disease are higher than those in general population; for the other, OSA contributes the impairs of renal function [2, 3]. Our previous studies indicated that cystatin C, a biomarker of early renal impairs, was higher in several OSA patients without complications [4], and continuous positive airway pressure (CPAP) treatment can normalize cystatin C levels in those patients [5]. The potential mechanisms of OSA-related renal impair are inconclusive. Previous studies elucidated that OSA leads to renal impairs thorough hypertension, sympathetic nervous system and renin-angiotensin-aldosterone system overactivation, endothelial dysfunction, and increased oxidative stress [6, 7].
Apoptosis and autophagy are two important cellular processes with complex and intersecting networks. Evidence shows that hypoxia is closely related with both apoptosis and autophagy [8, 9]. Chronic intermittent hypoxia (CIH) is a novel pathophysiological hallmark of OSA [1]. A study by Liu and colleagues [10] concluded that CIH induces differential expression of miRNAs which was associated with apoptosis or autophagy-related gene expression. Previous studies also showed that CIH enhances both hippocampal and myocardial apoptosis in rat mimicking OSA, and telmisartan administration can relieve the apoptosis levels [11, 12]. Autophagy in hippocampal neurons can be also aggravated by intermittent hypoxia (IH) [13]. However, data addressing the effect of CIH on apoptosis and autophagy levels in the kidney are scarce.
The present study aims to evaluate the effect of CIH on renal apoptosis and autophagy and further to assess the influence of telmisartan on those in a mouse model.
Materials and method
Animals and subgroups
Thirty-six 7-week-old male C57BL/6 mice were purchased from Chinese Academy of Science Laboratory Animals Center in Shanghai, China. Mice were kept in a departmental animal facility on a 12:12-hour light-dark cycle, and free access to water and food, and were randomly assigned to the following groups (n = 12 in each group): normoxia, chronic intermittent hypoxia (CIH), and intermittent hypoxia plus telmisartan (CIH+temisartan). The body weights of mice in each group were measured every week. This protocol was approved by the ethics committee in Zhongshan Hospital, Xiamen University, and conducted in accordance with the Guide for the Care and Use of Laboratory Animals [14].
Chronic intermittent hypoxia exposure
The protocol of chronic intermittent hypoxia exposure was based on previous studies [15, 16]. Briefly, mice in the CIH and CIH+temisartan groups (n = 24) were placed in a plexiglass chamber with one-way valves, and a programmable instrument which regulated the flow of oxygen, nitrogen, and compressed air into the chamber. This system ensured the oxygen concentration in the chamber varying from 21 to nadir 5–7%. The cycle time of hypoxia and reoxygenation is 2 min. The experimental period of CIH exposure was from 08:00 AM to 04:00 PM daily for 12 consecutive weeks.
Drug administration
Mice in CIH+telmisartan group were administered telmisartan (10 mg/kg dissolved in double-distilled water), while mice in the normoxia and CIH groups were administrated equal volume of double-distilled water. The drug administration by oral gavage was from the third week to the end of the CIH exposure.
Tissue preparation
After 12 weeks of the experimentation, mice were anesthetized with pentobarbital and exsanguinated by cardiac puncture. Blood samples were collected and centrifuged at 3000g for 15 min at 4 °C; then, the supernatants (serum) were collected and stored at − 80 °C for future analysis. Kidneys were either frozen in liquid nitrogen then transferred to − 80 °C refrigerator for further analysis or fixed in buffered 10% formalin for histological examination and immunohistochemistry. Kidney tissues were homogenized by RIPA lysis buffer (Beyotime, Beijing, China), then centrifuged at − 4 °C. After the supernatants were extracted, the protein concentrations were detected with bicinchoninic acid protein assay (Beyotime, Beijing, China); then, the supernatants were preserved in − 80 °C refrigerator.
Detection of blood urea nitrogen, serum creatinine, and serum and kidney angiotensin II concentration
Blood urea nitrogen and serum creatinine were analyzed using a Hitachi 7020 automatic analyzer (Hitachi Co. Ltd., Tokyo, Japan). The levels of angiotensin II in serum and kidney homogenates were determined by enzyme-linked immunosorbent assay (ELISA) using mouse angiotensin II immunoassay kit according to the instruction of the manufacturer (RayBiotech, Inc., USA).
Hematoxylin and eosin staining and TUNEL assay
Kidney tissue of each group in the formalin was further embedded in paraffin and sectioned in 5-μm slices. Slices were stained with hematoxylin and eosin (HE). A commercially available terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining kit (Roch, China) was used to detect apoptosis according to the manufacturer’s instructions. The numbers of apoptotic cells and total cell in the visual field (× 400 magnification) were determined. The results are presented as the percentage of apoptotic cells among the total cell population. Leica DM2500 microscope was used for photographing.
Western blotting
The supernatants of kidney tissue were used for analyzing the expression of special proteins. The protein expressive levels of angiotensin II receptor type 1 (AGTR1), Bax, caspase-3, autophagy-related protein LC3, and beclin-1 were evaluated by western blotting. Equal protein (40 μg/band) from the supernatants of each group were subjected to 12% SDS-PAGE and transferred to PVDF membranes. Membranes were blocked in 5% non-fat dry milk in TBST (10 Mm Tris-HCL, pH 7.5, 150 Mm NaCL, 0.05% Tween-20) for 1 hour at room temperature. After washing by TBST for three times, membranes were incubated with rabbit-anti-AGTR1 (Novus Biologicals, Littleton, CO, USA), rabbit-anti-Bax (Cell Signaling Technology, Beverly, MA, USA), rabbit anti-caspase-3 (Cell Signaling Technology, Beverly, MA, USA), rabbit anti-beclin-3 (Cell Signaling Technology, Beverly, MA, USA), rabbit anti-LC3 (Novus Biologicals, Littleton, CO, USA), and mouse anti-β-actin (Santa Cruz Biotechnology, USA) overnight at 4 °C. After incubated with a secondary antibody conjugated to horseradish peroxidase at room temperature for 1 hour, membranes were developed and exposed using an enhanced chemiluminescence kit (Clarity™ Western ECL Substrate, Bio-Rad). The band densities on the membranes were estimated using the ImageJ analysis software (National Institutes of Health, Bethesda, MD, USA). All experiments were repeated triplicate.
Statistical analysis
GraphPad Prism software 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used to assess the data. All data are shown as a mean ± standard deviation and compared using one-way analysis of variance and further post hoc test for further comparison between groups. A p value less than 0.05 was considered as statistical significance.
Results
Change in body weight, blood urea nitrogen, serum creatinine, and angiotensin II in each group
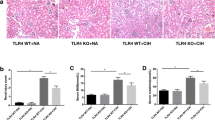
Mice in normoxia group (25.64 ± 1.59 g) gained more body weight than those in the CIH (23.05 ± 0.95 g) and CIH+telmisartan (21.15 ± 1.15 g) groups at the twelfth week of experimentation (p < 0.001) (Fig. 1). No difference in body weight between the CIH and CIH+telmisartan groups (p > 0.05) (Table 1). No significant differences in blood urea nitrogen and serum creatinine between groups. When compared with the normoxia and CIH groups, increased serum and kidney tissue angiotensin II levels were detected in the CIH+telmisartan group (p < 0.001) (Table 1). Compared with the normoxia group, the expression of AGTR1 was higher in the CIH group. After telmisartan administration, its expression decreased (Fig. 2).
Western blotting for AGTR1 protein expression. The AGTR1 protein levels in the CIH group significantly increased when compared to the normoxia group (p < 0.05). Treatment with telmisartan obviously decreased the AGTR1 expression in kidney tissue (compared to the CIH group, p < 0.001). AGTR1 angiotensin II receptor type 1, CIH chronic intermittent hypoxia
Kidney histopathological changes
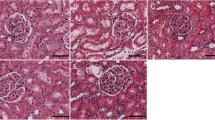
To investigate whether CIH and telmisartan influence the kidney architecture, a histopathological analysis of kidney tissue stained by hematoxylin and eosin were performed. After reviewing × 100 and × 400 magnified images, no abnormal architecture of the glomerular and tubular was detected in all groups (Fig. 3).
Effect of telmisartan on apoptosis in mice subjected to the CIH
TUNEL staining results depicted that the percentage of apoptotic cells, mostly tubular cells, in the CIH group was significantly higher than that of the normoxia group. After treatment with telmisartan, the apoptosis rate was decreased significantly (Fig. 4A). Both Bax and cleaved caspase-3 were increased in the CIH group than in normoxia group, while these protein levels were decreased in mice receiving telmisartan treatment (Fig. 4B, C).
Effect of telmisartan on apoptosis induced by CIH. The percentage of apoptotic cells (mostly tubular cells) was significantly higher in the CIH group than that of the normoxia group. After treating with telmisartan, the apoptotic percentage in the CIH+telmisartan group was lower than that of the CIH group (A). Mice in the CIH group had higher Bax (B) and cleaved caspase-3 (C) protein levels than those in the normoxia group. In comparison to the CIH group, these proteins levels were decreased in the CIH+telmisartan group (B, C). ***p < 0.001 when compared with normoxia group; *p < 0.05 when compared with normoxia group; ###p < 0.001 when compared with the CIH group; ##p < 0.01 when compared with the CIH group. CIH chronic intermittent hypoxia
Effect of telmisartan on autophagy in mice subjected to the CIH
Compared to mice exposed to normoxia, the expressions of autophagy-related proteins, beclin-1 and LC3, were significantly increased in mice subjected to the CIH. After receiving telmisartan treatment, these protein levels were decreased (Fig. 5).
Effect of telmisartan on autophagy induced by CIH. Western blotting results showed that the beclin-1 levels were higher in the CIH group than those of normoxia group, while the levels were decreased after mice received telmisartan treatment (Fig. 5 A, B); CIH induced high expression of LC3, while telmisartan can attenuate LC3 expression (Fig. 5 C, D). ***p < 0.001 when compared with normoxia group; ##p < 0.01 when compared with the CIH group; ###p < 0.001 when compared with the CIH group. CIH chronic intermittent hypoxia
Discussion
The present study demonstrated that CIH accelerates the progression of apoptosis and autophagy in kidney tissue of a mouse model mimicking OSA. We further found that telmisartan attenuates the CIH-induced apoptosis and autophagy.
The relationship between sleep apnea and kidney impair/disease is confirmed by several previous studies [3,4,5, 17,18,19,20]. A population-based cohort study in Taiwan demonstrated that patients with sleep apnea had a 1.94-fold and 2.2-fold increase in the incidences of chronic kidney disease and end-stage renal disease [18]. Our previous study showed that cystatin C, a novel biomarker of kidney impair, was increased in patients with an apnea-hypopnea index of more than 30 events/hour [4]. A systematic review and meta-analysis concluded the significant association between OSA and higher albuminuria/proteinuria and a lower estimated glomerular filtration rate [19]. A study indicates that CPAP treatment can improve renal hemodynamics and the downregulation of the renal renin-angiotensin system in OSA patients [21]. Previous experimental studies indicated that the potential mechanisms of OSA induced renal impairs include overactivation of the sympathetic nervous system and renal-angiotensin system, high oxidative stress and inflammation levels, endothelial dysfunction, and high glomerular perfusion [20, 22,23,24]. Intermittent hypoxia also contributes to histological kidney damage and high growth factor expression in an mouse model mimicking OSA [25].
Both apoptosis and autophagy are common in subjects with renal injury [26,27,28,29]. Hypoxia causes renal injury partly through apoptosis and autophagy [26, 30]. A study by Wu and co-authors found that CIH results in tubular endothelial apoptosis, and soluble receptor for advanced glycation end products can ameliorate the apoptosis levels. As far as author’s knowledge, few studies focus on the renal apoptosis and autophagy levels in the CIH or OSA subjects. This study demonstrated that CIH induces renal apoptosis and autophagy in a mouse model of OSA. It is postulated that the high CIH-induced apoptosis and autophagy levels are a pathogenesis of OSA enhancing the progression of renal impairs.
As an angiotensin II type 1 receptor blocker (ARB), telmisartan is a widely useful anti-hypertensive drug. Accumulated evidence proved that ARB attenuates CIH-related organ damage [11, 12, 31,32,33,34,35,36]. An experimental study from our group illustrated that telmisartan protects cardiocytes against CIH-induced oxidative stress [31]. The previous study showed that pre-CIH telmisartan administration ameliorates myocardial injury by attenuating CIH-induced myocardial apoptosis [12]. Meanwhile, the CIH-induced hippocampal apoptosis also can be attenuated by telmisartan via regulating nitric oxide synthase activity, inhibiting nitric oxide product, and decreasing lipid peroxidation and inflammatory response [11]. The present study with a mouse model of the CIH (12-week exposure of intermittent hypoxia) found that the renin-angiotensin-aldosterone system (RAAS) was blocked by telmisartan as previous studies showed [37, 38]. Furthermore, we proved that telmisartan administration ameliorates both apoptosis and autophagy levels in renal tissue. We speculated that telmisartan has the renal protective function in OSA patients, and it is suitable for OSA patients with hypertension or cardiovascular diseases.
Several limitations of this study should be noted. Firstly, normoxia+telmisartan group should have been appropriate, since apoptosis and autophagy levels could be also affected by telmisartan administration in normoxia condition. Secondly, only HE staining was conducted to evaluate the effect of the CIH and telmisartan on kidney architecture change, transmission electron microscopy should be performed to observe the ultrastructural change of kidney in a future study. Thirdly, similar to previous several studies [11, 12, 31, 37], omission of blood pressure measurement was a major limitation of our study. We postulated that not only improving the apoptosis and autophagy levels but also a possible lowering of blood pressure could telmisartan relieve CIH-induced kidney damage. Further experimental study is expected to classify the relationship between CIH, blood pressure change, and kidney damage. Finally, the potential biomechanisms which CIH leads to renal tubular apoptosis and autophagy and the function of telmisartan were not under discussion in the present study.
Conclusions
The present study elucidated that both apoptosis and autophagy levels were increased in kidney tissue of a CIH mouse model. Telmisartan ameliorates both levels. It is postulated that telmisartan can be considered as a potential drug for reliving the renal impairs in OSA patients thorough attenuating renal apoptosis and autophagy levels.
Change history
26 June 2020
In the article that appeared on Page: 341-348, Vol 23 (15 September 2018) of the Sleep and breathing [1], one error was discovered in Figure 3. The picture of Normoxia and CIH in 100X is the same one. The corrected version of Figure 3 is presented here.
References
Jordan AS, McSharry DG, Malhotra A (2014) Adult obstructive sleep apnoea. Lancet 383(9918):736–747. https://doi.org/10.1016/S0140-6736(13)60734-5
Marrone O, Bonsignore MR (2018) Obstructive sleep apnea and chronic kidney disease: open questions on a potential public health problem. J Thorac Dis 10(1):45–48. https://doi.org/10.21037/jtd.2017.12.12
Marrone O, Battaglia S, Steiropoulos P, Basoglu OK, Kvamme JA, Ryan S, Pepin JL, Verbraecken J, Grote L, Hedner J, Bonsignore MR, the ESADA study group (2016) Chronic kidney disease in European patients with obstructive sleep apnea: the ESADA cohort study. J Sleep Res 25(6):739–745. https://doi.org/10.1111/jsr.12426
Zhang XB, Lin QC, Deng CS, Chen GP, Cai ZM, Chen H (2013) Elevated serum cystatin C in severe OSA younger men without complications. Sleep Breath 17(1):235–241. https://doi.org/10.1007/s11325-012-0678-2
Zhang XB, Jiang XT, Lin QC, Chen X, Zeng HQ (2014) Effect of continuous positive airway pressure on serum cystatin C among obstructive sleep apnea syndrome patients. Int Urol Nephrol 46(10):1997–2002. https://doi.org/10.1007/s11255-014-0779-x
Turek NF, Ricardo AC, Lash JP (2012) Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis 60(5):823–833. https://doi.org/10.1053/j.ajkd.2012.04.027
Sun W, Yin X, Wang Y, Tan Y, Cai L, Wang B, Cai J, Fu Y (2012) Intermittent hypoxia-induced renal antioxidants and oxidative damage in male mice: hormetic dose response. Dose Response 11(3):385–400. https://doi.org/10.2203/dose-response.12-027.Cai
Zhou Q, Peng X, Liu X, Chen L, Xiong Q, Shen Y, Xie J, Xu Z, Huang L, Hu J, Wan R, Hong K (2018) FAT10 attenuates hypoxia-induced cardiomyocyte apoptosis by stabilizing caveolin-3. J Mol Cell Cardiol 116:115–124. https://doi.org/10.1016/j.yjmcc.2018.02.008
Qiao L, Fu J, Xue X, Shi Y, Yao L, Huang W et al (2018) Neuronalinjury and roles of apoptosis and autophagy in a neonatal rat model of hypoxia-ischemia-induced periventricular leukomalacia. Mol Med Rep 17(4):5940–5949. https://doi.org/10.3892/mmr.2018.8570
Liu KX, Chen GP, Lin PL, Huang JC, Lin X, Qi JC, Lin QC (2018) Detection and analysis of apoptosis- and autophagy-related miRNAs of mouse vascular endothelial cells in chronic intermittent hypoxia model. Life Sci 193:194–199. https://doi.org/10.1016/j.lfs.2017.11.001
Yuan X, Guo X, Deng Y, Zhu D, Shang J, Liu H (2015) Chronic intermittent hypoxia-induced neuronal apoptosis in the hippocampus is attenuated by telmisartan through suppression of iNOS/NO and inhibition of lipid peroxidation and inflammatory responses. Brain Res 1596:48–57. https://doi.org/10.1016/j.brainres.2014.11.035
Yuan X, Zhu D, Guo XL, Deng Y, Shang J, Liu K, Liu HG (2015) Telmisartan attenuates myocardial apoptosis induced by chronic intermittent hypoxia in rats: modulation of nitric oxide metabolism and inflammatory mediators. Sleep Breath 19(2):703–709. https://doi.org/10.1007/s11325-014-1081-y
Song S, Tan J, Miao Y, Zhang Q (2017) Effect of different levels of intermittent hypoxia on autophagy of hippocampal neurons. Sleep Breath 21(3):791–798. https://doi.org/10.1007/s11325-017-1512-7
National Research Concil (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the care and use of laboratory animals. National Academies Press (US), Washington, DC
Chen XY, Zeng YM, Zhang YX, Wang WY, Wu RH (2013) Effect of chronic intermittent hypoxia on theophylline metabolism in mouse liver. Chin Med J 126(1):118–123
Zhang XB, Zeng YM, Chen XY, Zhang YX, Ding JZ, Xue C (2018) Decreased expression of hepatic cytochrome P450 1A2 (CYP1A2) in a chronic intermittent hypoxia mouse model. J Thorac Dis 10(2):825–834. https://doi.org/10.21037/jtd.2017.12.106
Xu J, Yoon IY, Chin HJ (2016) The effect of sleep apnea on all-cause mortality in nondialyzed chronic kidney disease patients. Sleep Med 27-28:32–38. https://doi.org/10.1016/j.sleep.2016.07.026
Lee YC, Hung SY, Wang HK, Lin CW, Wang HH, Chen SW, Chang MY, Ho LC, Chen YT, Liou HH, Tsai TC, Tseng SH, Wang WM, Lin SH, Chiou YY (2015) Sleep apnea and the risk of chronic kidney disease: a nationwide population-based cohort study. Sleep 38(2):213–221. https://doi.org/10.5665/sleep.4400
Hwu DW, Lin KD, Lin KC, Lee YJ, Chang YH (2017) The association of obstructive sleep apnea and renal outcomes—a systematic review and meta-analysis. BMC Nephrol 18(1):313. https://doi.org/10.1186/s12882-017-0731-2
Abuyassin B, Sharma K, Ayas NT, Laher I (2015) Obstructive sleep apnea and kidney disease: a potential bidirectional relationship? J Clin Sleep Med 11(8):915–924. https://doi.org/10.5664/jcsm.4946
Nicholl DD, Hanly PJ, Poulin MJ, Handley GB, Hemmelgarn BR, Sola DY et al (2014) Evaluation of continuous positive airway pressure therapy on renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med 190(5):572–580. https://doi.org/10.1164/rccm.201403-0526OC
Lyons OD, Bradley TD, Chan CT (2015) Hypervolemia and sleep apnea in kidney disease. Semin Nephrol 35(4):373–382. https://doi.org/10.1016/j.semnephrol.2015.06.008
Poonit ND, Zhang YC, Ye CY, Cai HL, Yu CY, Li T, Cai XH (2017) Chronic intermittent hypoxia exposure induces kidney injury in growing rats. Sleep Breath 22:453–461. https://doi.org/10.1007/s11325-017-1587-1
Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K, Bohm M (2012) Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension 60(1):172–178. https://doi.org/10.1161/HYPERTENSIONAHA.112.191965
Abuyassin B, Badran M, Ayas NT, Laher I (2018) Intermittent hypoxia causes histological kidney damage and increases growth factor expression in a mouse model of obstructive sleep apnea. PLoS One 13(2):e0192084. https://doi.org/10.1371/journal.pone.0192084
Liu LL, Li D, He YL, Zhou YZ, Gong SH, Wu LY et al (2017) miR-210 protects renal cell against hypoxia-induced apoptosis by targeting HIF-1 alpha. Mol Med 23:1. https://doi.org/10.2119/molmed.2017.00013
Xie Y, Jiang D, Xiao J, Fu C, Zhang Z, Ye Z et al (2018) Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway. Cell Death Dis 9(3):338. https://doi.org/10.1038/s41419-018-0358-7
Havasi A, Borkan SC (2011) Apoptosis and acute kidney injury. Kidney Int 80(1):29–40. https://doi.org/10.1038/ki.2011.120
Livingston MJ, Dong Z (2014) Autophagy in acute kidney injury. Semin Nephrol 34(1):17–26. https://doi.org/10.1016/j.semnephrol.2013.11.004
Wang IK, Sun KT, Tsai TH, Chen CW, Chang SS, Yu TM, Yen TH, Lin FY, Huang CC, Li CY (2015) MiR-20a-5p mediates hypoxia-induced autophagy by targeting ATG16L1 in ischemic kidney injury. Life Sci 136:133–141. https://doi.org/10.1016/j.lfs.2015.07.002
Wang WY, Zeng YM, Chen XY, Zhang YX (2013) Effect of telmisartan on local cardiovascular oxidative stress in mouse under chronic intermittent hypoxia condition. Sleep Breath 17(1):181–187. https://doi.org/10.1007/s11325-012-0669-3
Knight WD, Saxena A, Shell B, Nedungadi TP, Mifflin SW, Cunningham JT (2013) Central losartan attenuates increases in arterial pressure and expression of FosB/DeltaFosB along the autonomic axis associated with chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 305(9):R1051–R1058. https://doi.org/10.1152/ajpregu.00541.2012
Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ (2010) Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol 171(1):36–45. https://doi.org/10.1016/j.resp.2010.02.003
Marcus NJ, Philippi NR, Bird CE, Li YL, Schultz HD, Morgan BJ (2012) Effect of AT1 receptor blockade on intermittent hypoxia-induced endothelial dysfunction. Respir Physiol Neurobiol 183(2):67–74. https://doi.org/10.1016/j.resp.2012.05.025
Ren J, Liu W, Deng Y, Li GC, Pan YY, Xie S, Jin M, Liu HG (2017) Losartan attenuates aortic endothelial apoptosis induced by chronic intermittent hypoxia partly via the phospholipase C pathway. Sleep Breath 21(3):679–689. https://doi.org/10.1007/s11325-017-1479-4
Fenik VB, Singletary T, Branconi JL, Davies RO, Kubin L (2012) Glucoregulatory consequences and cardiorespiratory parameters in rats exposed to chronic-intermittent hypoxia: effects of the duration of exposure and losartan. Front Neurol 3:51. https://doi.org/10.3389/fneur.2012.00051
Wang W, Song A, Zeng Y, Chen X, Zhang Y, Shi Y et al (2018) Telmisartan protects chronic intermittent hypoxic mice via modulating cardiac renin-angiotensin system activity. BMC Cardiovasc Disord 18(1):133. https://doi.org/10.1186/s12872-018-0875-4
Chen C, Zhang Z, Li Z, Zhang F, Peng M, Chen Y, Wang Y (2014) Losartan attenuates microvascular permeability in mechanical ventilator-induced lung injury in diabetic mice. Mol Biol Rep 41(2):809–814. https://doi.org/10.1007/s11033-013-2920-9
Acknowledgments
This work was supported by Grant 3502Z20154019 for Fund from Xiamen Science and Technology Bureau, Grant 2018-2-65 for Youth Research Fund from Fujian Provincial Health Bureau, and Grant 2018J01393 for Fund from Natural Science Foundation of Fujian Province, China.
Author information
Authors and Affiliations
Contributions
Conception and design: X-B Zhang, J-H Cai, and H-Q Zeng. Collection and assembly of data: X-B Zhang, X Cheng, and M Wang. Data analysis and interpretation: X-B Zhang, YY Yang, X-B Luo, and H-Q Zeng. Manuscript writing: all authors. Manuscript revising: XB Zhang, YY Yang, HC Ewurum. Final approval of manuscript: all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhang, XB., Cai, JH., Yang, YY. et al. Telmisartan attenuates kidney apoptosis and autophagy-related protein expression levels in an intermittent hypoxia mouse model. Sleep Breath 23, 341–348 (2019). https://doi.org/10.1007/s11325-018-1720-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-018-1720-9