Abstract
Purpose
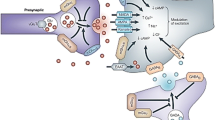
Metabotropic glutamate receptor 2 (mGluR2) has been implicated in various psychiatric and neurological disorders, such as schizophrenia and Alzheimer’s disease. We have previously developed [11C]7 as a PET radioligand for imaging mGluR2. Herein, [18F]JNJ-46356479 ([18F]8) was synthesized and characterized as the first 18F-labeled mGluR2 imaging ligand to enhance diagnostic approaches for mGluR2-related disorders.
Procedures
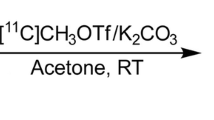
JNJ-46356479 (8) was radiolabeled via the copper (I)-mediated radiofluorination of organoborane 9. In vivo PET imaging experiments with [18F]8 were conducted first in C57BL/6 J mice and Sprague-Dawley rats to obtain whole body biodistribution and brain uptake profile. Subsequent PET studies were done in a cynomolgus monkey (Macaca fascicularis) to investigate the uptake of [18F]8 in the brain, its metabolic stability, as well as pharmacokinetic properties.
Results
JNJ-46356479 (8) exhibited excellent selectivity against other mGluRs. In vivo PET imaging studies showed reversible and specific binding characteristic of [18F]8 in rodents. In the non-human primate, [18F]8 displayed good in vivo metabolic stability, excellent brain permeability, fast and reversible kinetics with moderate heterogeneity across brain regions. Pre-treatment studies with compound 7 revealed time-dependent decrease of [18F]8 accumulation in mGluR2 rich regions based on SUV values with the highest decrease in the nucleus accumbens (18.7 ± 5.9%) followed by the cerebellum (18.0 ± 7.9%), the parietal cortex (16.9 ± 7.8%), and the hippocampus (16.8 ± 6.9%), similar to results obtained in the rat studies. However, the volume of distribution (VT) results derived from 2T4k model showed enhanced VT from a blocking study with compound 7. This is probably because of the potentiating effect of compound 7 as an mGluR2 PAM as well as related non-specific binding in the tissue data.
Conclusions
[18F]8 readily crosses the blood-brain barrier and demonstrates fast and reversible kinetics both in rodents and in a non-human primate. Further investigation of [18F]8 on its binding specificity would warrant translational study in human.






Similar content being viewed by others
References
Tanabe Y, Masu M, Ishii T, Shigemoto R, Nakanishi S (1992) A family of metabotropic glutamate receptors. Neuron 8:169–179
Cartmell J, Schoepp DD (2000) Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75:889–907
Chaki S (2010) Group II metabotropic glutamate receptor agonists as a potential drug for schizophrenia. Eur J Pharmacol 639:59–66
Downing AM, Kinon BJ, Millen BA Zhang L, Liu L, Morozova MA, Brenner B, Rayle TJ, Nisenbaum L, Zhao F, Gomez JC (2014) A double-bind, placebo-controlled comparator study of LY2140023 monohydrate in patients with schizophrenia. BMC Psychiatry 14:351
Feyissa AM, Woolverton WL, Miguel-Hidalgo JJ, Wang Z, Kyle PB, Hasler G, Stockmeier CA, Iyo AH, Karolewicz B (2010) Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry 34:279–283
Conn PJ, Jones CK (2009) Promise of mGluR2/3 activators in psychiatry. Neuropsychopharmacol 34:248–249
Muguruza C, Meana JJ, Callado LF (2016) Group II metabotropic glutamate receptors as targets for novel antipsychotic drugs. Front Pharmacol 7:130
Mazzitelli M, Palazzo E, Maione S, Neugebauer V (2018) Group II Metabotropic Glutamate receptors: role in pain mechanisms and pain modulation. Front Mol Neurosci 11:383
Richards G, Messer J, Faull RLM, Stadler H, Wichmann J, Huguenin P, Bohrmann B, Mutel V (2010) Altered distribution of mGlu2 receptors in β-amyloid-affected brain regions of Alzheimer cases and aged PS2APP mice. Brain Res 1363:180–190
Caraci F, Molinaro G, Battaglia G et al (2011) Targeting group II metabotropic glutamate (mGlu) receptors for the treatment of psychosis associated with Alzheimer's disease: selective activation of mGlu2 receptors amplifies β-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol Pharmacol 79:618–626
Leurquin-Sterk G, Celen S, Van Laere K, Koole M, Bormans G, Langlois X , Van Hecken A, Te Riele P, Alcázar J, Verbruggen A, de Hoon J, Andrés J-I, Schmidt ME (2017) What we observe in vivo is not always what we see in vitro: development and validation of 11C-JNJ-42491293, A novel radioligand for mGluR2. J Nucl Med 58:110–116
Andres JI, Alcazar J, Cid JM, De Angelis M, Iturrino L, Langlois X, Lavreysen H, Trabanco AA, Celen S, Bormans G (2012) Synthesis, evaluation, and radiolabeling of new potent positive allosteric modulators of the metabotropic glutamate receptor 2 as potential tracers for positron emission tomography imaging. J Med Chem 55:8685–8699
Lohith T, McQuade P, Salinas C et al (2016) First-in-human PET imaging of mGluR2 receptors. J Nucl Med 57:213
McQuade P, Joshi A, Miller P et al (2016) Discovery and preclinical evaluation of an mGluR2-NAM PET radioligand. J Nucl Med 57:290
Zhang X, Kumata K, Yamasaki T, Cheng R, Hatori A, Ma L, Zhang Y, Xie L, Wang L, Kang HJ, Sheffler DJ, Cosford NDP, Zhang MR, Liang SH (2017) Synthesis and preliminary studies of a novel negative allosteric modulator, 7-((2,5-dioxopyrrolidin-1-yl)methyl)-4-(2-fluoro-4-[11C]methoxyphenyl)quinoline-2-carboxamide, for imaging of metabotropic glutamate receptor 2. ACS Chem Neurosci 8:1937–1948
Kumata K, Hatori A, Yamasaki T, Zhang Y, Mori W, Fujinaga M, Xie L, Nengaki N, Zhang MR (2019) Synthesis and evaluation of 4-(2-fluoro-4-[11C]methoxyphenyl)-5-((2-methylpyridin-4-yl)methoxy)picolinamide for PET imaging of the metabotropic glutamate receptor 2 in the rat brain. Bioorg Med Chem 27:483–491
Zhang X, Zhang Y, Chen Z, Shao T, van R, Kumata K, Deng X, Fu H, Yamasaki T, Rong J, Hu K, Hatori A, Xie L, Yu Q, Ye W, Xu H, Sheffler DJ, Cosford NDP, Shao Y, Tang P, Wang L, Zhang MR, Liang SH (2020) Synthesis and preliminary studies of 11C-labeled tetrahydro-1,7-naphthyridine-2-carboxamides for PET imaging of metabotropic glutamate receptor 2. Theranostics 10:11178–11196
Li Z, Krause S, Suzuki M, Sasaki T (2016) Radiotracer compounds. World patent WO 2016/033190 A1. 2016 Mar 3
Van Gool MLM, Andres-Gil JI, Alcazar-Vaca MJ, Bormans GMR, Celen SJL, Joost V (2016) Radiolabelled mGluR2 PET ligands. World patent WO2016/087489 A1
Yu M, Nagren K, Chen YI et al (2003) Radiolabeling and biodistribution of methyl 2-(methoxycarbonyl)-2-(methylamino) bicyclo [2.1.1]-hexane-5-carboxylate, a potent neuroprotective drug. Life Sci 73:1577–1585
Wang J-Q, Zhang Z, Kuruppu D, Brownell AL (2012) Radiosynthesis of PET radiotracer as a prodrug for imaging group II metabotropic glutamate receptors in vivo. Bioorg Med Chem Lett 22:1958–1962
Yuan G, Qu X, Zheng B, Neelamegam R, Afshar S, Iyengar S, Pan C, Wang J, Kang HJ, Ondrechen MJ, Poutiainen P, el Fakhri G, Zhang Z, Brownell AL (2020) Design, synthesis, and characterization of benzimidazole derivatives as positron emission tomography imaging ligands for metabotropic glutamate receptor 2. J Med Chem 63:12060–12072
Cid JM, Tresadern G, Vega JA, de Lucas AI, del Cerro A, Matesanz E, Linares ML, García A, Iturrino L, Pérez-Benito L, Macdonald GJ, Oehlrich D, Lavreysen H, Peeters L, Ceusters M, Ahnaou A, Drinkenburg W, Mackie C, Somers M, Trabanco AA (2016) Discovery of 8-trifluoromethyl-3-cyclopropylmethyl-7-[(4-(2,4-difluorophenyl)-1-piperazinyl)methyl]-1,2,4-triazolo[4,3-a]pyridine (JNJ-46356479), a selective and orally bioavailable mGlu2 receptor positive allosteric modulator (PAM). J Med Chem 59:8495–8507
Yuan G, Shoup TM, Moon SH, Brownell AL (2020) A concise method for fully automated radiosyntheses of [18F]JNJ-46356479 and [18F]FITM via Cu-mediated 18F-fluorination of organoboranes. RSC Adv 10:25223–25227
Hilton J, Yokoi F, Dannals RF, Ravert HT, Szabo Z, Wong DF (2000) Column-switching HPLC for the analysis of plasma in PET imaging studies. Nucl Med Biol 27:627–630
Collier T, Normandin M, El Fakhri G et al (2013) Automation of column-switching HPLC for analysis of radiopharmaceuticals and their metabolites in plasma. J Nucl Med 54:1133
Jenkinson M, Beckmann CF, Behrens TE et al (2012) Fsl. Neuroimage 62:782–790
Seidlitz J, Sponheim C, Glen D, Ye FQ, Saleem KS, Leopold DA, Ungerleider L, Messinger A (2018) A population MRI brain template and analysis tools for the macaque. Neuroimage 170:121–131
Paxinos G, Huang XF, Toga AW (1999) The rhesus monkey brain in stereotaxic coordinates. Academic Press, San Diego
McLaren DG, Kosmatka KJ, Oakes TR et al (2009) A population-average MRI-based atlas collection of the rhesus macaque. Neuroimage 45:52–59
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539
Zischler J, Kolks N, Modemann D, Neumaier B, Zlatopolskiy BD (2017) Alcohol-enhanced Cu-mediated radiofluorination. Chem-Eur J 23:3251–3256
Ohishi H, Neki A, Mizuno N (1998) Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci Res 30:65–82
O'Brien DE, Shaw DM, Cho HP et al (2018) Differential pharmacology and binding of mGlu2 receptor allosteric modulators. Mol Pharmacol 93:526–540
Doornbos ML, Perez-Benito L, Tresadern G et al (2016) Molecular mechanism of positive allosteric modulation of the metabotropic glutamate receptor 2 by JNJ-46281222. Br J Pharmacol 173:588–600
Acknowledgment
mGluR1-6 and mGluR8 agonist and antagonist functional data as well as mGluR2 PAM activity were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # HHSN-271-2013-00017-C (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth (email to: bryan_roth@med.unc.edu) at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll (email to: jdrisco1@mail.nih.gov) at NIMH, Bethesda MD, USA. For experimental details please refer to the PDSP web site https://pdspdb.unc.edu/pdspWeb/ (https://pdspdb.unc.edu/pdspWeb/).
Funding
This project was financially supported by NIH grants [1R01EB021708 and 1R01NS100164] and the grants [1S10RR023452-01 and 1S10OD025234-01] for the imaging instrumentation and characterization of the organic compounds as well as the NIH grants [S10OD018035 and P41EB022544] to support the blood counting and metabolite analysis equipment used in the primate studies.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All animal experiments were approved and done under the guidelines of the Subcommittee on Research Animals of the Massachusetts General Hospital and Harvard Medical School in accordance with the Guide of NIH for the Care and Use of Laboratory Animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 115 kb)
Rights and permissions
About this article
Cite this article
Yuan, G., Guehl, N.J., Zheng, B. et al. Synthesis and Characterization of [18F]JNJ-46356479 as the First 18F-Labeled PET Imaging Ligand for Metabotropic Glutamate Receptor 2. Mol Imaging Biol 23, 527–536 (2021). https://doi.org/10.1007/s11307-021-01586-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01586-0