Abstract
Purpose
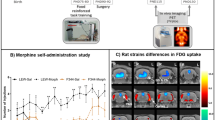
Non-medical use of prescription opioids, especially among adolescents, has been substantially increased in recent years. However, the neuromechanism remains largely unexplored. In the present study, we aimed to investigate the brain metabolic changes in adolescent rats following chronic escalating morphine administration using positron emission tomography (PET).
Procedures
2-Deoxy-2-[18F]Fluoro-d-glucose ([18F]FDG) microPET imaging was performed, and statistical parametric mapping (SPM) was used for image analysis. Glucose transporter 3 (Glut-3), dopamine D2 receptor (D2R), and Mμ-opioid receptor (μ-OR) were used for immunostaining analysis.
Results
Cerebral glucose metabolism was increased in the corpus callosum (CC) and right retrosplenial dysgranular cortex (rRSD), while it was decreased in the right ventral pallidum (rVP). The expressions of Glut-3, D2R, and μ-OR were increased in CC and rRSD, while they were decreased in rVP. Furthermore, glucose metabolism and Glut-3 expression were positively correlated with the expressions of D2R or μ-OR in CC, rRSD, and rVP.
Conclusions
[18F]FDG microPET brain imaging study in combination with immunohistological investigation revealed that CC, rRSD, and rVP were specifically involved in opioid dependence in adolescents. Our findings provided valuable insights into the neuromechanism of adolescent addiction of prescription opioids and might have important implications for the development of prevention and intervention approaches.






Similar content being viewed by others
References
Lake S, Milloy MJ, Dong H, Hayashi K, Wood E, Kerr T, DeBeck K (2016) Initiation into prescription opioid injection and associated trends in heroin use among people who use illicit drugs. Drug Alcohol Depen 169:73–79
Compton WM, Boyle M, Wargo E (2015) Prescription opioid abuse: problems and responses. Prev Med 80:5–9
Boyd CJ, McCabe SE, Cranford JA, Young A (2006) Adolescents' motivations to abuse prescription medications. Pediatrics 6:2472–2480
Kalivas PW, O'Brien C (2008) Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33:166–180
Silva-Rodríguez J, García-Varela L, López-Arias E, Domínguez-Prado I, Cortés J, Pardo-Montero J, Fernández-Ferreiro A, Ruibal Á, Sobrino T, Aguiar P (2016) Impact of benzodiazepines on brain FDG-PET quantification after single-dose and chronic administration in rats. Nucl Med Biol 43:827–834
Fowler JS, Volkow ND, Kassed CA, Chang L (2007) Imaging the addicted human brain. Sci Pract Perspect 3:4–16
Henry PK, Murnane KS, Votaw JR, Howell LL (2010) Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging Behav 4:212–219
Salmanzadeh H, Azizi H, Semnanian S (2017) Adolescent chronic escalating morphine administration induces long lasting changes in tolerance and dependence to morphine in rats. Physiol Behav 174:191–196
Zhu Y, Du R, Zhu Y et al (2013) PET mapping of neurofunctional changes in a post-traumatic stress disorder model. J Nucl Med 57:1474–1477
Xi W, Su D, Nie B, Yu Y, Shan B, Chen Q, Tian M, Zhang H (2013) 18F-FDG PET study reveals brain functional changes during attention in rats. J Nucl Med 54:1969–1973
Nie B, Chen K, Zhao S, Liu J, Gu X, Yao Q, Hui J, Zhang Z, Teng G, Zhao C, Shan B (2013) A rat brain MRI template with digital stereotaxic atlas of fine anatomical delineations in Paxinos space and its automated application in voxel-wise analysis. Hum Brain Mapp 34:1306–1318
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th ed. San Diego: Academic Press
Volkow ND, Fowler JS, Wang GJ (2003) The addicted human brain: insights from imaging studies. J Clin Invest 111:1444–1451
Edwards TJ, Sherr EH, Barkovich AJ, Richards LJ (2014) Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain 137:1579–1613
Vargas WM, Bengston L, Gilpin NW, Whitcomb BW, Richardson HN (2014) Alcohol binge drinking during adolescence or dependence during adulthood reduces prefrontal myelin in male rats. J Neurosci 34:14777–14782
Bi Y, Yuan K, Feng D, Xing L, Li Y, Wang H, Yu D, Xue T, Jin C, Qin W, Tian J (2015) Disrupted inter-hemispheric functional and structural coupling in internet addiction adolescents. Psychiatry Res 234:157–163
Lin F, Wu G, Zhu L, Lei H (2013) Heavy smokers show abnormal microstructural integrity in the anterior corpus callosum: a diffusion tensor imaging study with tract-based spatial statistics. Drug Alcohol Depen 129:82–87
Van Groen T, Wyss JM (1992) Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol 315:200–216
Zhang Y, Gong J, Xie C, Ye EM, Jin X, Song H, Yang Z, Shao Y (2015) Alterations in brain connectivity in three sub-regions of the anterior cingulate cortex in heroin-dependent individuals: evidence from resting state fMRI. Neuroscience 284:998–1010
Vann SD, Aggleton JP, Maguire EA (2009) What does the retrosplenial cortex do? Nat Rev Neurosci 10:792–802
Radwanska A, Debowska W, Liguz-Lecznar M, Brzezicka A, Kossut M, Cybulska-Klosowicz A (2010) Involvement of retrosplenial cortex in classical conditioning. Behav Brain Res 214:231–239
Canales JJ (2013) Deficient plasticity in the hippocampus and the spiral of addiction: focus on adult neurogenesis. Curr Top Behav Neurosci 15:293–312
Mickiewicz A, Dallimore J, Napier TC (2009) The ventral pallidum is critically involved in the development and expression of morphine-induced sensitization. Neuropsychopharmacology 34:874–886
Hubner CB, Koob GF (1990) The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Res 508:20–29
Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF (2005) The neural consequences of repeated cocaine exposure revealed by functional MRI in awake rats. Neuropsychopharmacology 30:936–943
Richard JM, Ambroggi F, Janak PH, Fields HL (2016) Ventral Pallidum neurons encode incentive value and promote cue-elicited instrumental actions. Neuron 90:1165–1173
Vannucci SJ (1994) Developmental expression of GLUT1 and GLUT3 glucose transporters in rat brain. J Neurochem 62:240
Piepponen TP, Kivastik T, Katajamäki J et al (1997) Involvement of opioid mu 1 receptors in morphine-induced conditioned place preference in rats. Pharmacol Biochem Behav 58:275–279
Acknowledgements
This work was partly sponsored by the National Natural Science Foundation of China (81301243,81425015), the Natural Science Foundation of Zhejiang Province (LY17H180004), the Research Fund for the Doctoral Program of Higher Education of China (20120101120031), the grants from the Health Bureau of Zhejiang Province (2014KYA095, WKJ2013-2-016), and supported by Zhejiang University K. P. Chao’s High Technology Development.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal studies were approved by the Zhejiang University Ethics Committee for Animal Experiments (Protocol No. ZJU2015-069-02).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, Q., Hou, H., Feng, J. et al. PET Imaging Reveals Brain Metabolic Changes in Adolescent Rats Following Chronic Escalating Morphine Administration. Mol Imaging Biol 20, 993–1000 (2018). https://doi.org/10.1007/s11307-018-1188-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-018-1188-9