Abstract
Purpose
The effects of deep brain stimulation (DBS) have been studied primarily by cellular studies, which lack the ability to elucidate DBS-related responses on a whole-brain scale. 2-Deoxy-2-[18F]fluoro-d-glucose positron emission tomography ([18F]FDG-PET) reflects changes in neural activity throughout the entire brain volume. The aim of this study was to investigate the whole-brain effect of DBS on the glucose utilization in healthy rats.
Procedures
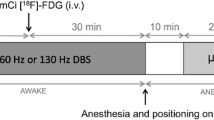
Seven rats were implanted with a DBS electrode in the right hippocampus and injected with [18F]FDG to measure the glucose metabolism during DBS.
Results
Analysis reveals significant DBS-induced decreases in the glucose metabolism in the bilateral hippocampus and other limbic structures.
Conclusions
This study demonstrates that DBS exhibits not only a local effect around the electrode tip but also in other limbic regions. [18F]FDG-PET studies have the potential to provide better insight into the mechanism of action of DBS by simultaneously observing activity at multiple sites in the brain.







Similar content being viewed by others
References
Okun MS (2012) Deep-brain stimulation for Parkinson’s disease. N Engl J Med 367:1529–1538
Bronstein JM, Tagliati M, Alterman RL et al (2011) Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 68(2):165–171
Birdno MJ, Grill WM (2008) Mechanisms of deep brain stimulation in movement disorders as revealed by changes in stimulus frequency. Neurotherapeutics 5:14–25
Benabid AL (2003) Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol 13:696–706
Fischer R, Salanova V, Witt T, SANTE Study Group (2010) Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51(5):899–908
Boon P, Raedt R, De Herdt V et al (2009) Electrical stimulation for the treatment of epilepsy. Neurotherapeutics 6:218–277
Vonck K, Sprengers M, Carrette E et al (2013) A decade of experience with deep brain stimulation for patients with refractory medial temporal lobe epilepsy. Int J Neural Syst 23(1):1–13
Nuttin B, Cosyns P, Demeulemeester H et al (1999) Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet 354:1526
Abelson JL, Curtis GC, Sagher O et al (2005) Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry 75(5):510–516
Lai HY, Albaugh DL, Kao Y-CJ et al (2014) Robust deep brain stimulation functional MRI procedures in rats and mice using an MR-compatible tungsten microwire electrode. Magn Reson Med. doi:10.1002/mrm.25239
Younce JR, Albaugh DL, Shih Y-YL (2014) Deep brain stimulation with simultaneous FMRI in rodents. J Vis Exp 84:e51271
Kerklaan BJ, Berckel BN, Herholz K et al (2014) The added value of 18-Fluorodeoxyglucose-positron emission tomography in the diagnosis of the behavioral variant of frontotemporal dementia. Am J Alzheimers Dis Dement. doi:10.1177/1533317514524811
Jueptner M, Weiller C (1995) Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage 2(2):148–156
Magistretti PJ, Pellerin L (1996) The contribution of astrocytes to the 18F-2-deoxyglucose signal in PET activation studies. Mol Psychiatry 1(6):445–452
Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14(6):724–738
Hershey T, Revilla FJ, Wernle AR et al (2003) Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology 61(6):816–821
Asanuma K, Tang C, Ma Y et al (2006) Network modulation in the treatment of Parkinson’s disease. Brain 129:2667–2678
Trost M, Su S, Su P et al (2006) Network modulation by the subthalamic nucleus in the treatment of Parkinson’s disease. Neuroimage 31:301–307
Goerendt K, Lawrence AD, Mehta MA et al (2006) Distributed neural actions of anti-parkinsonian therapies as revealed by PET. J Neural Transm 113:75–86
Karimi M, Golchin N, Tabbal SD et al (2008) Subthalamic nucleus stimulation-induced regional blood flow responses correlate with improvement of motor signs in Parkinson disease. Brain 131:2710–2719
Geday J, Ostergaard K, Johnsen E, Gjedde A (2009) STN-stimulation in Parkinson’s disease restores striatal inhibition of thalamocortical projection. Hum Brain Mapp 30:112–121
Borghammer P, Cumming P, Aanerud J, Gjedde A (2009) Artefactual subcortical hyperperfusion in PET studies normalized to global mean: lessons from Parkinson’s disease. Neuroimage 45:249–257
Kwan P, Brodie MJ (2000) Early identification of refractory epilepsy. N Engl J Med 342:314–319
Spencer SS (2002) Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia 43:219–227
Morrell M (2006) Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol 19:164–168
Theodore WH, Fischer R (2007) Brain stimulation for epilepsy. Acta Neurochir Suppl 97:261–272
Wyckhuys T, De Smedt T, Claeys P et al (2007) High frequency deep brain stimulation in the hippocampus modifies seizure characteristics in kindled rats. Epilepsia 48:1543–1550
Wyckhuys T, Boon P, Raedt R (2010) Suppression of hippocampal epileptic seizures in the kainate rat by Poisson distributed stimulation. Epilepsia 51(11):2297–2304
Cuellar-Herrera M, Neri-Bazan L, Rocha (2006) Behavioral effects of high frequency electrical stimulation of the hippocampus on electrical kindling in rats. Epilepsy Res 72:10–17
Velasco AL, Fischer RS (2014) Electrical brain stimulation for epilepsy. Nat Rev Neurol 10:261–270
Velasco AL, Velasco F, Velasco M et al (2007) Electrical stimulation of the hippocampal epileptic foci for seizure control: a double-blind, long-term follow-up study. Epilepsia 48:1895–1903
Velasco F, Velasco M, Velasco AL et al (2001) Electrical stimulation for epilepsy: stimulation of hippocampal foci. Stereotact Funct Neurosurg 77:223–227
Vonck K, Boon P, Achten E et al (2002) Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol 52:556–565
Osorio I, Frei MG, Sunderam S et al (2005) Automated seizure abatement in humans using electrical stimulation. Ann Neurol 57:258–268
Boon P, Vonck K, De Herdt V et al (2007) Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia 48:1551–1560
Cuckiert A, Cuckiert CM, Burattini JA, Lima AM (2014) Seizure outcome after hippocampal deep brain stimulation in a prospective cohort of patients with refractory temporal lobe epilepsy. Seizure 23(1):6–9
Goodman JH, Berger RE, Tcheng TK (2005) Preemptive low-frequency stimulation decreases the incidence of amygdala-kindled seizures. Epilepsia 46:1–7
Akman T, Erken H, Acar G et al (2011) Effects of the hippocampal deep brain stimulation on cortical epileptic discharges in penicillin-induced epilepsy model in rats. Turk Neurosurg 21(1):1–5
Paxinos G, Watson C (2007) In: The rat brain in stereotactic coordinates, 6th edn. Academic Press
Wyckhuys T, Staelens S, Van Nieuwenhuyse B et al (2010) Hippocampal deep brain stimulation induces decreased rCBF in the hippocampal formation of the rat. Neuroimage 52(1):55–61
Rondouin G, Lerner-Natoli M, Hashizume A (1987) Wet dog shakes in limbic versus generalized seizures. Exp Neurol 95(2):500–505
Press WH, Flannery BP, Teukolsky SA, Vetterling WT (1992) Direction set (Powell’s) methods in multidimensions. In: Numerical recipes in C, 2nd edn. Cambridge University Press, New York, pp 412–420
Becerra L, Pendse G, Chang PC et al (2011) Robust reproducible resting state networks in the awake rodent brain. PLoS One 6(10):e25701
Jonckers E, Van Audekerke J, De Visscher G et al (2011) Functional connectivity of the rodent brain: comparison of functional connectivity networks in rat and mouse. PLoS One 6(4):e18876
Buchhalter JR, Fieles A, Dichter MA (1990) Hippocampal commissural connections in the neonatal rat. Dev Brain Res 56:211–216
Klein J, Soto-Montenegro ML, Pascau J et al (2011) A novel approach to investigate neuronal network activity patterns affected by deep brain stimulation in rats. J Psychiatr Res 45:927–930
McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL (2004) Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 115:1239–1248
Canals S, Beyerlein M, Murayama Y, Logothetis NK (2008) Electric stimulation fMRI of the perforant pathway to the rat hippocampus. Magn Reson Imaging 26:978–986
Shih Y-YI, Yash TV, Rogers B, Duong TQ (2014) FMRI of deep brain stimulation at the rat ventral posteromedial thalamus. Brain Stimulation 7(2):190–193
Chao T-HH, Chen J-H, Yen C-T (2014) Repeated BOLD-fMRI imaging of deep brain stimulation responses in rats. PLoS ONE 9(5):e97305
van Welie I, van Hooft JA, Wadman WJ (2004) Homeostatic scaling of neuronal excitability by synaptic modulation of somatic hyperpolarization-activated I-h channels. Proc Natl Acad Sci U S A 101:5123–5128
Beurrier C, Bioulac B, Audin J, Hammond C (2001) High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 85(4):1351–1356
Vedam-Mai V, van Battum EY, Kamphuis W et al (2012) Deep brain stimulation and the role of astrocytes. Mol Psychiatry 17:124–131
Volman V, Ben-Jacob E, Levine H (2007) The astrocyte as a gatekeeper of synaptic information transfer. Neural Comput 19:303–326
Sibson NR (1998) Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A 95:316–321
Magistretti PJ, Pellerin L (1999) Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond Ser B 354:1155–1163
Dalsgaard MK, Ide K, Cai Y et al (2002) The intent to exercise influences the cerebral O(2)/carbohydrate uptake ratio in humans. J Physiol 540:681–689
Pellerin L, Bouzier-Sore AK, Aubert A et al (2007) Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55(12):1251–1262
Braitenberg V, Schüz A (1998) Density of neurons and synapses. In: Cortex: statistics and geometry of neuronal connectivity, 2nd edn. Springer, Berlin Heidelberg, pp 23–39
Waldvogel D (2000) The relative metabolic demand of excitation and inhibition. Nature 406:995–998
Weeks RA, Gerloff C, Dalakas M, Hallett M (1999) PET study of visually and non-visually guided finger movements in patients with severe pan-sensory neuropathies and healthy controls. Exp Brain Res 128:291–302
Turrigiano GG, Nelson SB (2004) Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5:97–107
Neves G, Cooke SF, Bliss TV (2008) Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci 9(1):65–75
Benazzouz A, Gao DM, Ni ZG et al (2000) Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience 99(2):289–295
van den Heuvel P, Mandl RCW, Kahn RS, Hulshoff Pol HE (2009) Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 30(10):3127–3141
Damoiseaux JS, Rombouts SA, Barkhof F et al (2006) Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 103:13848–13853
Chang SY, Kim I, Marsh MP (2012) Wireless fast-scan cyclic voltammetry to monitor adenosine in patients with essential tremor during deep brain stimulation. Mayo Clin Proc 87(8):760–765
Lyons MK (2011) Deep brain stimulation: current and future clinical applications. Mayo Clin Proc 86(7):662–672
Schiffer WK, Mirrione MM, Biegon A et al (2006) Serial microPET measures of the metabolic reaction to a microdialysis probe implant. J Neurosci Methods 155(2):272–284
van Luijtelaar G, Huang L (2013) Brain computer interface for epilepsy treatment—recent progress and future prospects. In: InTech open science—open minds 239–252. doi:10.5772/55800
Acknowledgments
This work is funded by iMinds and Ghent University. Prof. Dr. Roel Van Holen is supported by the Research Foundation, Flanders, Belgium (FWO). Prof. Dr. Christian Vanhove is supported by the GROUP-ID consortium of Ghent University. Prof. Dr. P. Boon is supported by grants from FWO and grants from BOF and by the Clinical Epilepsy Grant from Ghent University Hospital.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Den Berge, N., Keereman, V., Vanhove, C. et al. Hippocampal Deep Brain Stimulation Reduces Glucose Utilization in the Healthy Rat Brain. Mol Imaging Biol 17, 373–383 (2015). https://doi.org/10.1007/s11307-014-0801-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0801-9