Abstract
Purpose
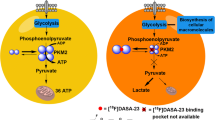
The mTOR kinase inhibitor AZD8055 inhibits both mTORC1 and mTORC2 leading to disruption of glucose metabolism and proliferation pathways. This study assessed the impact of single and multiple doses of AZD8055 on the uptake of the glucose metabolism marker 2-deoxy-2-[18 F]fluoro-d-glucose ([18 F]FDG) and the proliferation marker 3′-deoxy-3′-[18 F]fluorothymidine ([18 F]FLT) in U87-MG glioma xenografts.
Procedures
Mice bearing U87-MG tumours received either vehicle or AZD8055 (20 mg/kg) once daily p.o. Mice were imaged with either [18 F]FDG or [18 F]FLT PET to assess treatment response. Comparisons were made between in vivo imaging and ex vivo histopathology data.
Results
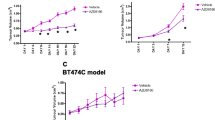
Tumour uptake of [18 F]FDG was reduced by 33 % 1 h after a single dose of AZD8055 and by 49 % following 4 days of dosing. These changes coincided with suppression of the mTOR pathway biomarkers pS6 and pAKT. In contrast, the effect of AZD8055 on [18 F]FLT uptake was inconsistent.
Conclusions
The very rapid change in [18 F]FDG uptake following acute AZD8055 treatment suggests that this could be used as an early mechanistic biomarker of metabolic changes resulting from mTOR inhibition. The utility of [18 F]FLT for measuring the anti-proliferative effect of AZD8055 remains unclear.




Similar content being viewed by others
References
García-Martínez JM, Alessi DR (2008) mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J 416:375–385
O'Reilly KE, Rojo F, She QB et al (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500–1508
Cloughesy TF, Yoshimoto K, Nghiemphu P et al (2008) Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med 5:e8
Marshall G, Howard Z, Dry J et al (2011) Benefits of mTOR kinase targeting in oncology: pre-clinical evidence with AZD8055. Biochem Soc Trans 39:456–459
Sylvie M, Guichard ZH et al (2012) AZD2014, a dual mTORC1 and mTORC2 inhibitor is differentiated from allosteric inhibitors of mTORC1 in ER+ breast cancer. Cancer Res 72(8):Suppl 1
Smith GC, Ong WK, Rewcastle GW et al (2012) Effects of acutely inhibiting PI3K isoforms and mTOR on regulation of glucose metabolism in vivo. Biochem J 442:161–169
Eary JF, O’Sullivan F, Powitan Y, Chandhury KR et al (2002) Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med Mol Imaging 29:1149–1154
Weber WA. (2009) Assessing tumor response to therapy. J Nucl Med.;501S-10S.
Benz MR, Czernin J, Tap WD et al (2010) FDG-PET/CT imaging predicts histopathologic treatment responses after neoadjuvant therapy in adult primary bone sarcomas. Sarcoma. doi:10.1155/2010/143540
Shinto A, Nair N, Dutt A, Baghel NS (2008) Early response assessment in gastrointestinal stromal tumors with FDG PET scan 24 hours after a single dose of imatinib. Clin Nucl Med 33:486–487
Thomas GV, Tran C, Mellinghoff IK et al (2006) Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med 12:122–127
Cejka D, Kuntner C, Preusser M et al (2009) FDG uptake is a surrogate marker for defining the optimal biological dose of the mTOR inhibitor everolimus in vivo. British J Cancer 2(100):1739–1745
Wei LH, Su H, Hildebrandt IJ et al (2008) Changes in tumor metabolism as readout for mammalian target of rapamycin kinase inhibition by rapamycin in glioblastoma. Clin Cancer Res 14:3416–3426
Ma WW, Jacene H, Song D et al (2009) [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. Clin Oncol 1(27):2697–2704
Troost EG, Bussink J, Hoffmann AL et al (2010) 18F-FLT PET/CT for early response monitoring and dose escalation in oropharyngeal tumors. J Nucl Med 51:866–874
Solit D, Santos E, Pratilas DA et al (2007) 3-Deoxy-3-[18 F]fluorothymidine positron emission tomography is a sensitive method for imaging the response of BRAF dependent tumors to MEK inhibition. Cancer Res 67:11463–11469
Benz MR, Czernin J, Allen-Auerbach MS et al (2012) 3′-Deoxy-3′[18F]fluorothymidine positron emission tomography for response assessment in soft tissue sarcoma: a pilot study to correlate imaging findings with tissue thymidine kinase 1 and Ki-67 activity and histopathologic response. Cancer 118:3135–3144
Workman P, Twentyman P, Balkwill F et al (1998) United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) guidelines for the welfare of animals in experimental neoplasia (Second edition). Br J Cancer 77:1–10
Keen H, Pichler B, Kukuk D et al (2012) An evaluation of 2-deoxy-2-[18F]fluoro-d-glucose and 3′-deoxy-3′-[18F]-fluorothymidine uptake in human tumor xenograft models. Mol Imaging Biol 14:355–365
Bao Q, Newport D, Chen M et al (2009) Performance evaluation of the Inveon dedicated PET preclinical tomograph based on the NEMA NU-4 standards. J Nucl Med 50:401–408
Gambhir S. Quantitative assay development for PET. Chapter 2 in Phelps, Michael E (Ed.) PET: molecular imaging and its biological applications. Springer; 2004
Chresta CM, Davies BR, Hickson I et al (2010) AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res 1(70):288–298
Lee SC, Marzec M, Liu X et al (2012) Decreased lactate concentration and glycolytic enzyme expression reflect inhibition of mTOR signal transduction pathway in B-cell lymphoma. NMR Biomed. doi:10.1002/nbm.2825
Nguyen QD, Perumal M, Waldman TA, Aboagye EO (2011) Glucose metabolism measured by [18F]fluorodeoxyglucose positron emission tomography is independent of PTEN/AKT status in human colon carcinoma cells. Transl Oncol 4:241–248
Aide N, Kinross K, Cullinane C et al (2010) 18F-FLT PET as a surrogate marker of drug efficacy during mTOR inhibition by everolimus in a preclinical cisplatin-resistant ovarian tumor model. J Nucl Med 51:1559–1564
McKinley ET, Ayers GD, Smith RA et al (2013) Limits of [18F]-FLT PET as a biomarker of proliferation in oncology. PLoS One 8:e58938
Soloviev D, Lewis D, Honess D, Aboagye E (2012) [18 F]FLT: an imaging biomarker of tumour proliferation for assessment of tumour response to treatment. Eur J Cancer 48:416–424
Chalkidou A, Landau DB, Odell EW et al (2012) Correlation between Ki-67 immunohistochemistry and 18 F-fluorothymidine uptake in patients with cancer: a systematic review and meta-analysis. Eur J Cancer 48:3499–3513. doi:10.1016/j.ejca.2012.05.001
Waterton JC, Pylkkanen L (2012) Qualification of imaging biomarkers for oncology drug development. Eur J Cancer 48:409–415
Patlak CS, Blasberg RG (1985) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5:584–590
Cheebsumon P, Velasquez LM, Hoekstra CJ et al (2011) Measuring response to therapy using FDG PET: semi-quantitative and full kinetic analysis. Eur J Nucl Med Mol Imaging 38:832–842
Maynard J, Ricketts SA, Gendrin C et al (2013) 2-Deoxy-2-[18 F]fluoro-d-glucose positron emission tomography demonstrates target inhibition with the potential to predict anti-tumour activity following treatment with the AKT inhibitor AZD5363. Mol Imaging Biol. Mol Imaging Biol 15:476–485
Schnell CR, Stauffer F, Allegrini PR et al (2008) Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer Res 68:6598–6607
Saito K, Matsumoto S, Yasui H et al (2012) Longitudinal imaging studies of tumor microenvironment in mice treated with the mTOR inhibitor rapamycin. PLoS One 7:e49456. doi:10.1371/journal.pone.0049456
Guimaraes AR, Ross R, Figuereido JL et al (2011) MRI with magnetic nanoparticles monitors downstream anti-angiogenic effects of mTOR inhibition. Mol Imaging Biol 13h:314–320
Conflict of Interest
All authors are employees of AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keen, H.G., Ricketts, SA., Maynard, J. et al. Examining Changes in [18 F]FDG and [18 F]FLT Uptake in U87-MG Glioma Xenografts as Early Response Biomarkers to Treatment with the Dual mTOR1/2 Inhibitor AZD8055. Mol Imaging Biol 16, 421–430 (2014). https://doi.org/10.1007/s11307-013-0705-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-013-0705-0