Abstract
Introduction
Zonisamide is a new-generation anticonvulsant antiepileptic drug metabolized primarily in the liver, with subsequent elimination via the renal route.
Objectives
Our objective was to evaluate the utility of pharmacometabolomics in the detection of zonisamide metabolites that could be related to its disposition and therefore, to its efficacy and toxicity.
Methods
This study was nested to a bioequivalence clinical trial with 28 healthy volunteers. Each participant received a single dose of zonisamide on two separate occasions (period 1 and period 2), with a washout period between them. Blood samples of zonisamide were obtained from all patients at baseline for each period, before volunteers were administered any medication, for metabolomics analysis.
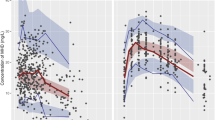
Results
After a Lasso regression was applied, age, height, branched-chain amino acids, steroids, triacylglycerols, diacyl glycerophosphoethanolamine, glycerophospholipids susceptible to methylation, phosphatidylcholines with 20:4 FA (arachidonic acid) and cholesterol ester and lysophosphatidylcholine were obtained in both periods.
Conclusion
To our knowledge, this is the only research study to date that has attempted to link basal metabolomic status with pharmacokinetic parameters of zonisamide.

Similar content being viewed by others
Change history
14 June 2018
The original version of this article contains a mistake.
References
Baker, M. (2011). Metabolomics: From small molecules to big ideas. Nature Methods, 8(2), 117–121.
Barr, J., Caballería, J., Martínez-Arranz, I., Domínguez-Díez, A., Alonso, C., Muntané, J., et al. (2012). Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. Journal of Proteome Research, 11(4), 2521–2532. https://doi.org/10.1021/pr201223p.
Borobia, A. M., Lubomirov, R., Ramírez, E., Lorenzo, A., Campos, A., Muñoz-Romo, R., et al. (2012). An acenocoumarol dosing algorithm using clinical and pharmacogenetic data in Spanish patients with thromboembolic disease. PLoS ONE, 7(7), e41360. https://doi.org/10.1371/journal.pone.0041360.
Brodie, M. J., Ben-Menachem, E., Chouette, I., & Giorgi, L. (2012). Zonisamide: Its pharmacology, efficacy and safety in clinical trials. Acta Neurologica Scandinavica, 126(S194), 19–28. https://doi.org/10.1111/ane.12016.
Committee for medicinal products for human use. (2010). Guideline on the investigation of bioequivalence (No. Doc. Ref.: CPMP/EWP/QWP/1401/98 Rev. 1/ Corr **). London. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf.
Duportet, X., Bastos, R., Aggio, M., Carneiro, S., & Villas-Bôas, S. G. (2012). The biological interpretation of metabolomic data can be misled by the extraction method used. Metabolomics, 8(3), 410–421.
Elbadawi-Sidhu, M., Baillie, R. A., Zhu, H., Chen, Y.-D. I., Goodarzi, M. O., Rotter, J. I., et al. (2017). Pharmacometabolomic signature links simvastatin therapy and insulin resistance. Metabolomics, 13(1), 11. https://doi.org/10.1007/s11306-016-1141-3.
Faught, E., Ayala, R., Montouris, G. G., Leppik, I. E., & Zonisamide 922 Trial Group, (2001). Randomized controlled trial of zonisamide for the treatment of refractory partial-onset seizures. Neurology, 57(10), 1774–1779.
Frampton, J. E., & Scott, L. J. (2005). Zonisamide: A review of its use in the management of partial seizures in epilepsy. CNS Drugs, 19(4), 347–367.
Friedman, J., Hastie, T., & Tibshirani, R. (2010). Regularization paths for generalized linear models via coordinate descent. Journal of Statistical Software. https://doi.org/10.18637/jss.v033.i01.
Fuhrer, T., & Zamboni, N. (2015). High-throughput discovery metabolomics. Current Opinion in Biotechnology, 31, 73–78. https://doi.org/10.1016/j.copbio.2014.08.006.
Gieser, G., Harigaya, H., Colangelo, P. M., & Burckart, G. (2011). Biomarkers in solid organ transplantation. Clinical Pharmacology and Therapeutics, 90(2), 217–220. https://doi.org/10.1038/clpt.2011.75.
Goodacre, R. (2007). Metabolomics of a superorganism. The Journal of Nutrition, 137(1 Suppl), 259S–266S.
Hastie, T., Tibshirani, R., & Friedman, J. H. (2009). The elements of statistical learning: Data mining, inference, and prediction (2nd ed.). New York: Springer.
Huang, Q., Aa, J., Jia, H., Xin, X., Tao, C., Liu, L., et al. (2015). A Pharmacometabonomic approach to predicting metabolic phenotypes and pharmacokinetic parameters of atorvastatin in healthy volunteers. Journal of Proteome Research, 14(9), 3970–3981. https://doi.org/10.1021/acs.jproteome.5b00440.
Indiveri, C., Iacobazzi, V., Tonazzi, A., Giangregorio, N., Infantino, V., Convertini, P., et al. (2011). The mitochondrial carnitine/acylcarnitine carrier: Function, structure and physiopathology. Molecular Aspects of Medicine, 32(4–6), 223–233. https://doi.org/10.1016/j.mam.2011.10.008.
Italiano, D., & Perucca, E. (2013). Clinical pharmacokinetics of new-generation antiepileptic drugs at the extremes of age: An update. Clinical Pharmacokinetics, 52(8), 627–645. https://doi.org/10.1007/s40262-013-0067-4.
Kaddurah-Daouk, R., & Weinshilboum, R. (2015). Metabolomic signatures for drug response phenotypes: Pharmacometabolomics enables precision medicine. Clinical Pharmacology & Therapeutics, 98(1), 71–75. https://doi.org/10.1002/cpt.134.
Kaneko, S., Okada, M., Hirano, T., Kondo, T., Otani, K., & Fukushima, Y. (1993). Carbamazepine and zonisamide increase extracellular dopamine and serotonin levels in vivo, and carbamazepine does not antagonize adenosine effect in vitro: Mechanisms of blockade of seizure spread. The Japanese Journal of Psychiatry and Neurology, 47(2), 371–373.
Kantae, V., Krekels, E. H. J., Esdonk, M. J., Van Lindenburg, P., Harms, A. C., Knibbe, C. A. J., et al. (2017). Integration of pharmacometabolomics with pharmacokinetics and pharmacodynamics: Towards personalized drug therapy. Metabolomics, 13(1), 9. https://doi.org/10.1007/s11306-016-1143-1.
Kito, M., Maehara, M., & Watanabe, K. (1996). Mechanisms of T-type calcium channel blockade by zonisamide. Seizure, 5(2), 115–119.
Kochak, G. M., Page, J. G., Buchanan, R. A., Peters, R., & Padgett, C. S. (1998). Steady-state pharmacokinetics of zonisamide, an antiepileptic agent for treatment of refractory complex partial seizures. Journal of Clinical Pharmacology, 38(2), 166–171.
Koves, T. R., Li, P., An, J., Akimoto, T., Slentz, D., Ilkayeva, O., et al. (2005). Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. The Journal of Biological Chemistry, 280(39), 33588–33598. https://doi.org/10.1074/jbc.M507621200.
Kurnaz, F. S., Hoffmann, I., & Filzmoser, P. (2017). Robust and sparse estimation methods for high dimensional linear and logistic regression. Retrieved from http://arxiv.org/abs/1703.04951.
Lenz, E. M., & Wilson, I. D. (2007). Analytical strategies in metabonomics. Journal of Proteome Research, 6(2), 443–458. https://doi.org/10.1021/pr0605217.
Leppik, I. E. (2004). Zonisamide: Chemistry, mechanism of action, and pharmacokinetics. Seizure, 13, S5–S9. https://doi.org/10.1016/j.seizure.2004.04.016.
Levy, R. H., Ragueneau-Majlessi, I., Garnett, W. R., Schmerler, M., Rosenfeld, W., Shah, J., & Pan, W.-J. (2004). Lack of a clinically significant effect of zonisamide on phenytoin steady-state pharmacokinetics in patients with epilepsy. Journal of Clinical Pharmacology, 44(11), 1230–1234. https://doi.org/10.1177/0091270004268045.
Lewerenz, J., Hewett, S. J., Huang, Y., Lambros, M., Gout, P. W., Kalivas, P. W., et al. (2013). The cystine/glutamate antiporter system x(c)(-) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxidants & Redox Signaling, 18(5), 522–555. https://doi.org/10.1089/ars.2011.4391.
Lin, Y. S., Kerr, S. J., Randolph, T., Shireman, L. M., Senn, T., & McCune, J. S. (2016). Prediction of intravenous busulfan clearance by endogenous plasma biomarkers using global pharmacometabolomics. Metabolomics, 12(10), 161. https://doi.org/10.1007/s11306-016-1106-6.
Martínez-Arranz, I., Mayo, R., Pérez-Cormenzana, M., Mincholé, I., Salazar, L., Alonso, C., & Mato, J. M. (2015). Enhancing metabolomics research through data mining. Journal of Proteomics, 127, 275–288. https://doi.org/10.1016/j.jprot.2015.01.019.
Masuda, Y., Karasawa, T., Shiraishi, Y., Hori, M., Yoshida, K., & Shimizu, M. (1980). 3-Sulfamoylmethyl-1,2-benzisoxazole, a new type of anticonvulsant drug. Pharmacological profile. Arzneimittel-Forschung, 30(3), 477–483.
McBean, G. J. (2002). Cerebral cystine uptake: A tale of two transporters. Trends in Pharmacological Sciences, 23(7), 299–302.
Mimaki, T., Suzuki, Y., Tagawa, T., Karasawa, T., & Yabuuchi, H. (1990). Interaction of zonisamide with benzodiazepine and GABA receptors in rat brain. Medical Journal of Osaka University, 39(1–4), 13–17.
Mizuno, K. (1997). Effects of carbamazepine and zonisamide on acetylcholine levels in rat striatum. Nihon shinkei seishin yakurigaku, 17(1), 17–23.
Muhrez, K., Benz-de Bretagne, I., Nadal-Desbarats, L., Blasco, H., Gyan, E., Choquet, S., et al. (2017). Endogenous metabolites that are substrates of organic anion transporter’s (OATs) predict methotrexate clearance. Pharmacological Research, 118, 121–132. https://doi.org/10.1016/j.phrs.2016.05.021.
Ojemann, L. M., Shastri, R. A., Wilensky, A. J., Friel, P. N., Levy, R. H., McLean, J. R., & Buchanan, R. A. (1986). Comparative pharmacokinetics of zonisamide (CI-912) in epileptic patients on carbamazepine or phenytoin monotherapy. Therapeutic Drug Monitoring, 8(3), 293–296.
Okada, M., Hirano, T., Kawata, Y., Murakami, T., Wada, K., Mizuno, K., et al. (1999). Biphasic effects of zonisamide on serotonergic system in rat hippocampus. Epilepsy Research, 34(2–3), 187–197.
Okada, M., Kaneko, S., Hirano, T., Ishida, M., Kondo, T., Otani, K., & Fukushima, Y. (1992). Effects of zonisamide on extracellular levels of monoamine and its metabolite, and on Ca2+ dependent dopamine release. Epilepsy Research, 13(2), 113–119.
Okada, M., Kaneko, S., Hirano, T., Mizuno, K., Kondo, T., Otani, K., & Fukushima, Y. (1995). Effects of zonisamide on dopaminergic system. Epilepsy Research, 22(3), 193–205.
Okada, M., Kawata, Y., Mizuno, K., Wada, K., Kondo, T., & Kaneko, S. (1998). Interaction between Ca2+, K+, carbamazepine and zonisamide on hippocampal extracellular glutamate monitored with a microdialysis electrode. British Journal of Pharmacology, 124(6), 1277–1285. https://doi.org/10.1038/sj.bjp.0701941.
Peters, D. H., & Sorkin, E. M. (1993). Zonisamide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in epilepsy. Drugs, 45(5), 760–787.
Phapale, P. B., Kim, S.-D., Lee, H. W., Lim, M., Kale, D. D., Kim, Y.-L., et al. (2010). An Integrative approach for identifying a metabolic phenotype predictive of individualized pharmacokinetics of tacrolimus. Clinical Pharmacology & Therapeutics, 87(4), 426–436. https://doi.org/10.1038/clpt.2009.296.
R Development Core Team. (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, http://www.R-project.org/. R Foundation for Statistical Computing, Vienna, Austria.
Rock, D. M., Macdonald, R. L., & Taylor, C. P. (1989). Blockade of sustained repetitive action potentials in cultured spinal cord neurons by zonisamide (AD 810, CI 912), a novel anticonvulsant. Epilepsy Research, 3(2), 138–143.
Romigi, A., Femia, E. A., Fattore, C., Vitrani, G., Di Gennaro, G., & Franco, V. (2015). Zonisamide in the management of epilepsy in the elderly. Clinical Interventions in Aging, 10, 931–937. https://doi.org/10.2147/CIA.S50819.
Schauf, C. L. (1987). Zonisamide enhances slow sodium inactivation in Myxicola. Brain Research, 413(1), 185–188.
Schelldorfer, J., Meier, L., & Bühlmann, P. (2011). GLMMLasso: An algorithm for high-dimensional generalized linear mixed models using l1-penalization. Journal of Computational and Graphical Statistics. https://doi.org/10.1080/10618600.2013.773239.
Schulze-Bonhage, A. (2010). Zonisamide in the treatment of epilepsy. Expert Opinion on Pharmacotherapy, 11(1), 115–126. https://doi.org/10.1517/14656560903468728.
Sills, G., & Brodie, M. (2007). Pharmacokinetics and drug interactions with zonisamide. Epilepsia, 48(3), 435–441. https://doi.org/10.1111/j.1528-1167.2007.00983.x.
Stekhoven, D. J., & Bühlmann, P. (2012). Missforest-non-parametric missing value imputation for mixed-type data. Bioinformatics, 28, 112–118. https://doi.org/10.1093/bioinformatics/btr597.
Suzuki, S., Kawakami, K., Nishimura, S., Watanabe, Y., Yagi, K., Seino, M., & Miyamoto, K. (1992). Zonisamide blocks T-type calcium channel in cultured neurons of rat cerebral cortex. Epilepsy Research, 12(1), 21–27.
Tan, G., Zhao, B., Li, Y., Liu, X., Zou, Z., Wan, J., et al. (2017). Pharmacometabolomics identifies dodecanamide and leukotriene B4 dimethylamide as a predictor of chemosensitivity for patients with acute myeloid leukemia treated with cytarabine and anthracycline. Oncotarget. https://doi.org/10.18632/oncotarget.20733.
Ueda, Y., Doi, T., Tokumaru, J., & Willmore, L. J. (2003). Effect of zonisamide on molecular regulation of glutamate and GABA transporter proteins during epileptogenesis in rats with hippocampal seizures. Molecular Brain Research, 116(1–2), 1–6.
Uno, H., Kurokawa, M., Masuda, Y., & Nishimura, H. (1979). Studies on 3-substituted 1,2-benzisoxazole derivatives. 6. Syntheses of 3-(sulfamoylmethyl)-1,2-benzisoxazole derivatives and their anticonvulsant activities. Journal of Medicinal Chemistry, 22(2), 180–183.
van den Berg, R. A., Hoefsloot, H. C., Westerhuis, J. A., Smilde, A. K., & van der Werf, M. J. (2006). Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genomics, 7(1), 142. https://doi.org/10.1186/1471-2164-7-142.
Witten, D. M., & Tibshirani, R. (2009). Covariance-regularized regression and classification for high dimensional problems. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 71(3), 615–636.
Zhu, H., Bogdanov, M. B., Boyle, S. H., Matson, W., Sharma, S., Matson, S., et al. (2013). Pharmacometabolomics of response to sertraline and to Placebo in major depressive disorder—Possible role for methoxyindole pathway. PLoS ONE, 8(7), e68283. https://doi.org/10.1371/journal.pone.0068283.
Zou, H., & Hastie, T. (2005). Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society, Series B, 67, 301–320.
Acknowledgements
The authors would like to thank Dr. Jennifer Kirwan for her valuable comments to the manuscript. Also to the scientific committee and the participants of MOVISS 2017 Bio&Data, Vorau, Austria, for their feedback in metabolomics and interest in our data.
Funding
The ZNS bioequivalence study was funded by Laboratorios Normon, Ronda de Valdecarrizo 6, 28760 Tres Cantos, Madrid, SPAIN. The research project has been cofinanced by the Ministerio de Economia y Competitividad within the INNPACTO program (IPT-2012-0576-090000) and by the European Regional Development Fund (ERDF “A way of making Europe”).
Author information
Authors and Affiliations
Contributions
JCM-A and AJCS and AMB designed the study. JF, PG, HYT provided the pharmacokinetics study. AGB, IG, ID and LD contributed with the metabolomics results interpretation. JCM-A, AGB and AMB, wrote the manuscript. JCM-A perform the statistical analysis. All authors revised and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martínez-Ávila, J.C., García Bartolomé, A., García, I. et al. Pharmacometabolomics applied to zonisamide pharmacokinetic parameter prediction. Metabolomics 14, 70 (2018). https://doi.org/10.1007/s11306-018-1365-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-018-1365-5